Suzhou Chung-Hwa Chemical&Pharmaceutical Industrial Co.,Ltd.(hereinafter referred to as Suzhou Chung-Hwa Chemical&Pharmaceutical) was established in April 1993, Yongan Road factory was officially opened in 2014, located in the Jiangnan water town - Suzhou National High-tech Industrial Development Zone, covering an area of about 60,000 square meters, is a national high-tech modern pharmaceutical enterprise invested and established by Cenra, integrating scientific research, production and sales, and is also one of the first batch of pharmaceutical enterprises in China to pass GMP certification.
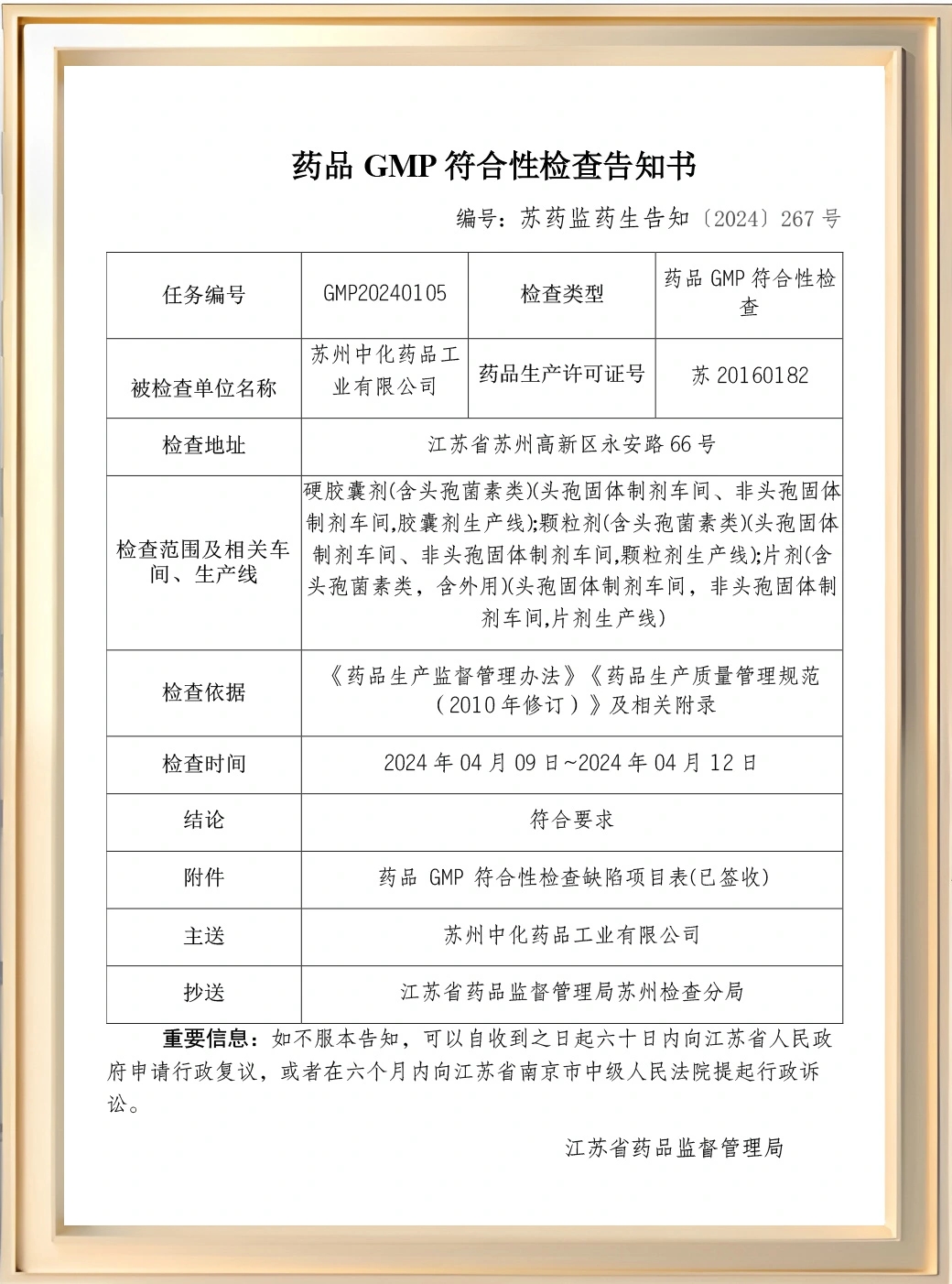
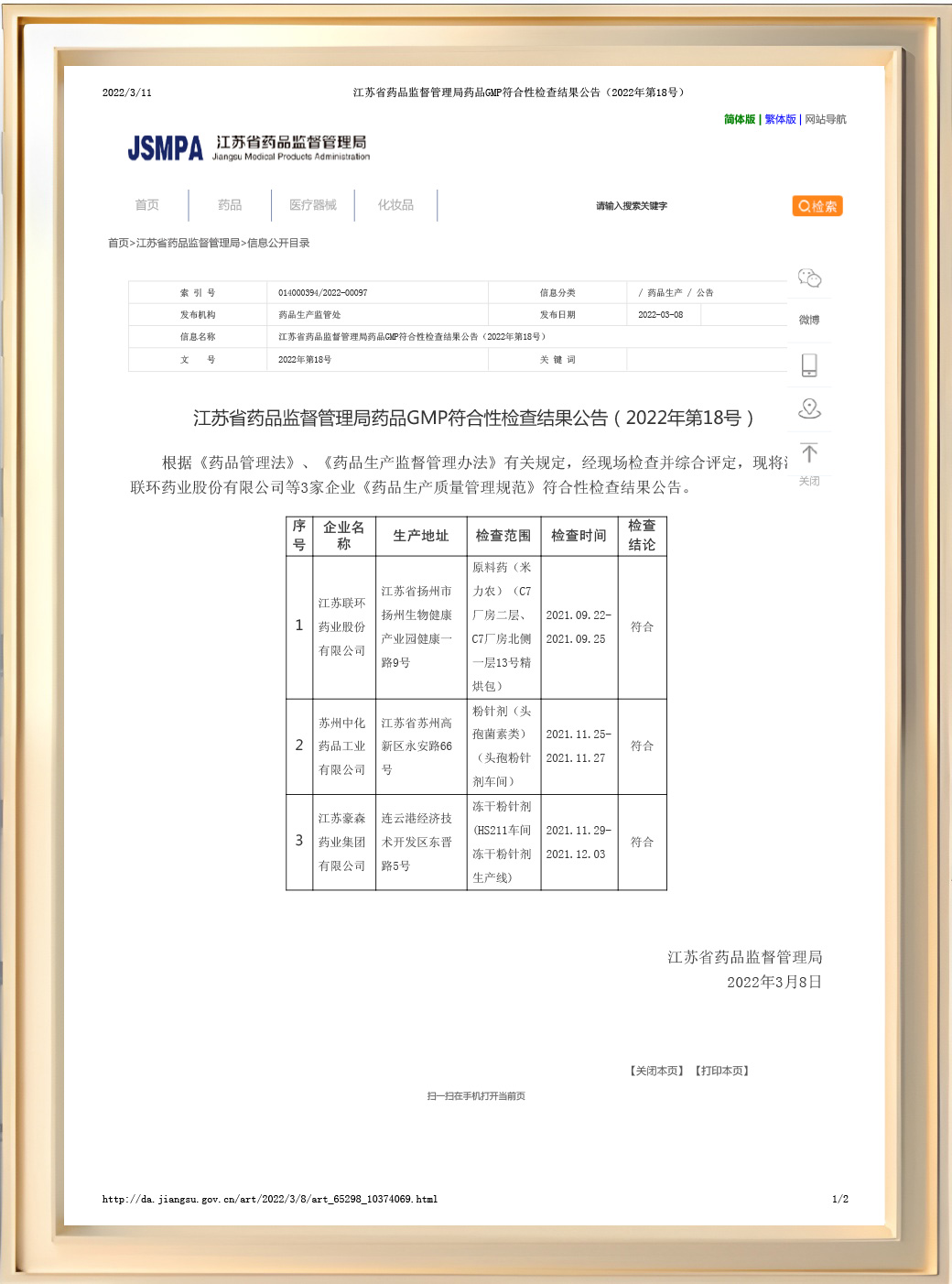
Suzhou Chung-Hwa Chemical&Pharmaceutical has established an independent and beautiful research building, built a preparation trial production workshop that meets GMP requirements, strived to build an independent R&D and innovation platform with an international vision, and established a cross-strait joint research institute with Cenra Research Institute, relying on Cenra's profound scientific research accumulation, adhering to the cutting-edge pharmaceutical concept and technological innovation orientation, and building core technology advantages. By equipping with the world's leading cutting-edge production equipment and intelligent inspection instruments, we fully implement GMP standards and precise quality control system.
Suzhou Chung-Hwa Chemical&Pharmaceutical has a sound sales network and after-sales service system, and the sales network covers the whole country, covering 31 provinces and cities and more than 1,000 counties and cities across the country. Marketing is committed to large-scale and diversified development, offline hospitals, pharmacies, and clinics, and its products cover more than 12,000 hospitals across the country. It has established good cooperative relations with well-reputable and powerful mainstream B2B e-commerce platforms such as "Pharmacist", "Yaojingcai" and "1 Yaocheng", and is committed to allowing more patients to use the high-quality drugs produced by Suzhou Chung-Hwa.
At present, the main drug categories include: digestive system drugs, cardiovascular system drugs, musculoskeletal system drugs, endocrine system drugs, anti-infective drugs and other functional nutrition products. In addition to the pharmaceutical field, Suzhou Chung-Hwa Pharmaceutical established a subsidiary, Suzhou Chung-Hwa Le Huo Jian Kang Co.,Ltd.. in 2021, and has its own brands such as MIAJACK and HEALTH CRANE, actively moving towards health fields such as personal care products and health food.
Nowadays, with the vigorous development of the Internet, Suzhou Chung-Hwa Chemical&Pharmaceutical has always adhered to the vision of being a "trustworthy health cause in the Chinese world", advocated the core values of "innovation, health, passion and care", and constantly innovated to keep up with the pace of the times. In addition to consolidating the existing professional drug production capacity and continuing to expand the scale, it also began to plan future cooperation with online and offline medical systems, aiming to build a smart technology health industry. No matter where you are in the world, Suzhou Chung-Hwa Chemical&Pharmaceutical will embrace health and happiness with you every day!
About Cenra lnc.
Founded in 1952, Cenra lnc. has been innovating and developing in the industry for more than 70 years, and has continuously improved the quality of its products and services. With many professional talents, it is one of the pharmaceutical factories with the most drug licenses, and it is also the most leading pharmaceutical factory in Taiwan's pharmaceutical industry.
Cenra lnc. has established many affiliated enterprises, such as Cenra API Solutions、Cenra Healthcare、Cenra Senior Care.; In addition to R&D and production of drugs, it also develops medical, health supplies, health services, and operation and marketing. On the basis of professional division of labor, it can also vertically integrate the resources of related enterprises, enter the latest technology, the latest drugs and the latest services into the world, and truly become the most trustworthy health cause!
 Fulexin® Cefuroxime Axetil Tablets
Fulexin® Cefuroxime Axetil Tablets Keluo® Cefaclor Capsules
Keluo® Cefaclor Capsules Kefu® Cefaclor Granules
Kefu® Cefaclor Granules Mieda® Cefradine Capsules
Mieda® Cefradine Capsules Lvxin® Cefalexin Capsules
Lvxin® Cefalexin Capsules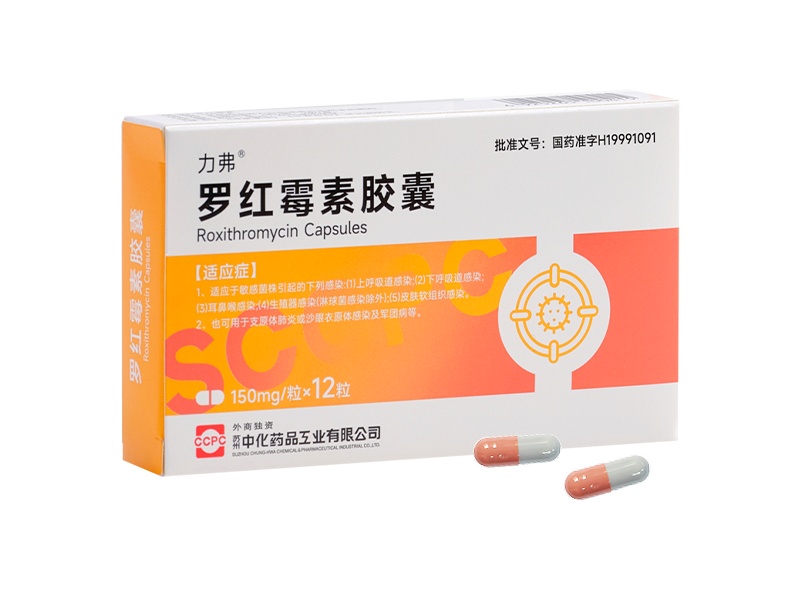 Lifu® Roxithromycin Capsules
Lifu® Roxithromycin Capsules Lifu® Roxithromycin Granules
Lifu® Roxithromycin Granules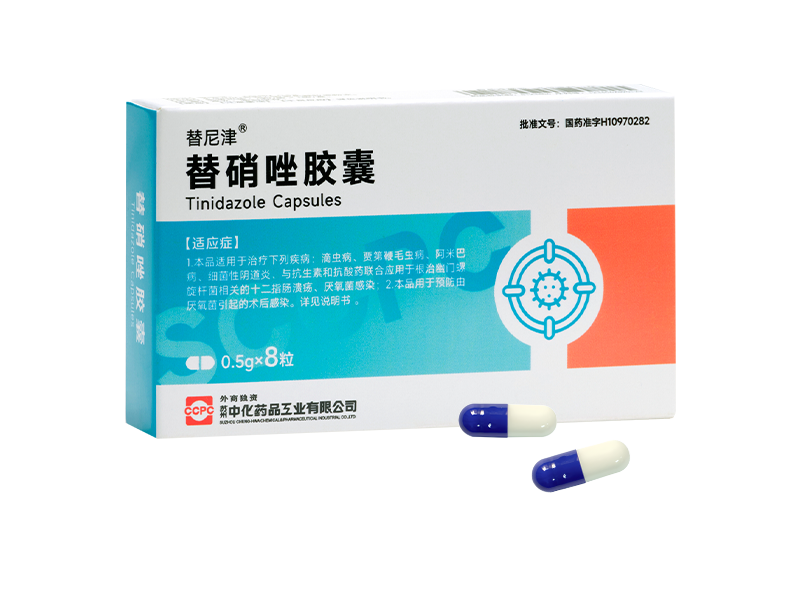 Tinijin® Tinidazole Capsules
Tinijin® Tinidazole Capsules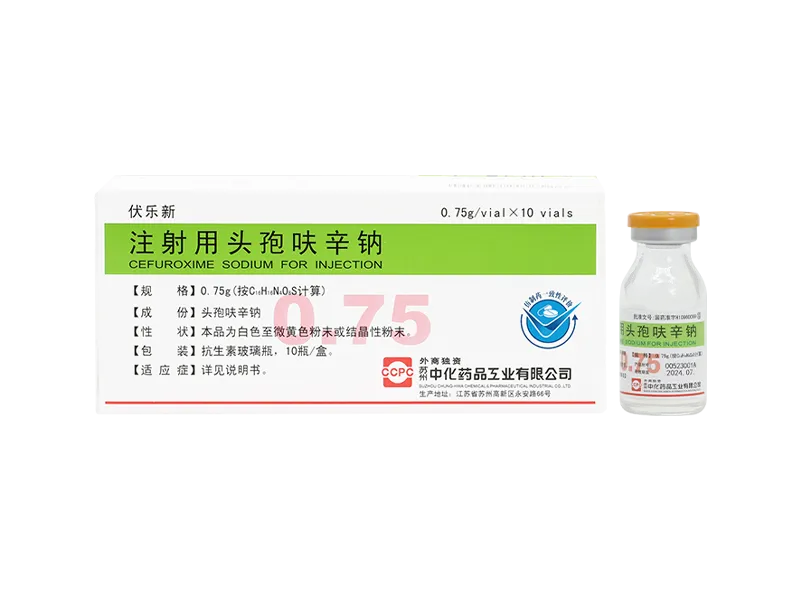 Fulexin® Cefazolin Sodium for Injection
Fulexin® Cefazolin Sodium for Injection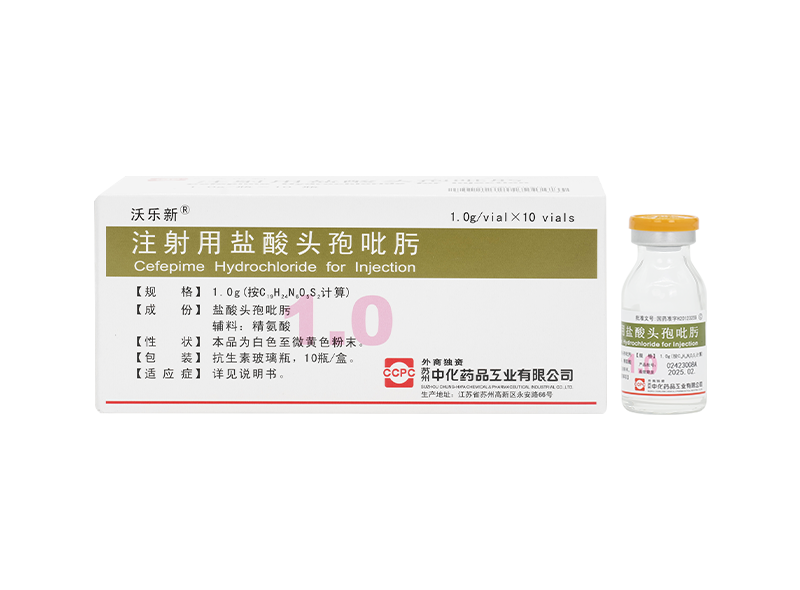 Wolexin® Cefepime Hydrochloride for Injection
Wolexin® Cefepime Hydrochloride for Injection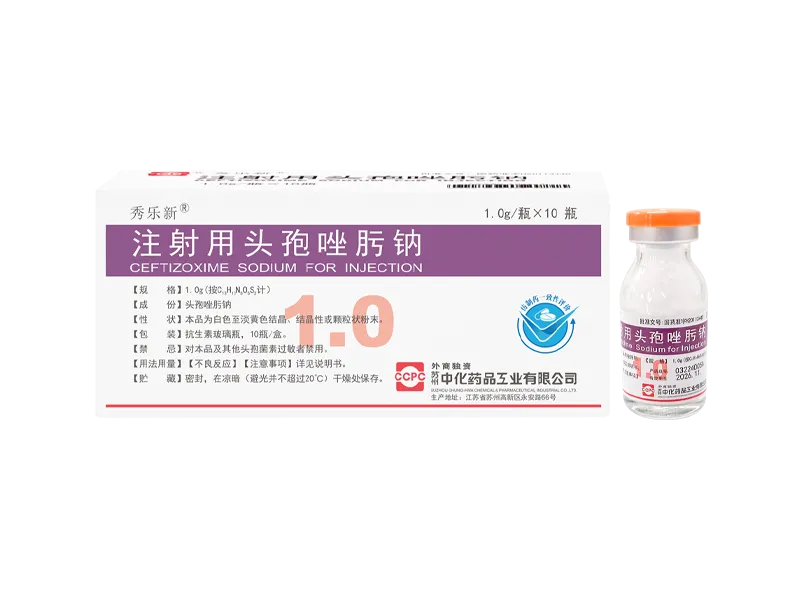 Xiulexin® Ceftizoxime Sodium for Injection
Xiulexin® Ceftizoxime Sodium for Injection Xinlexin® Ceftriaxone Sodium for Injection
Xinlexin® Ceftriaxone Sodium for Injection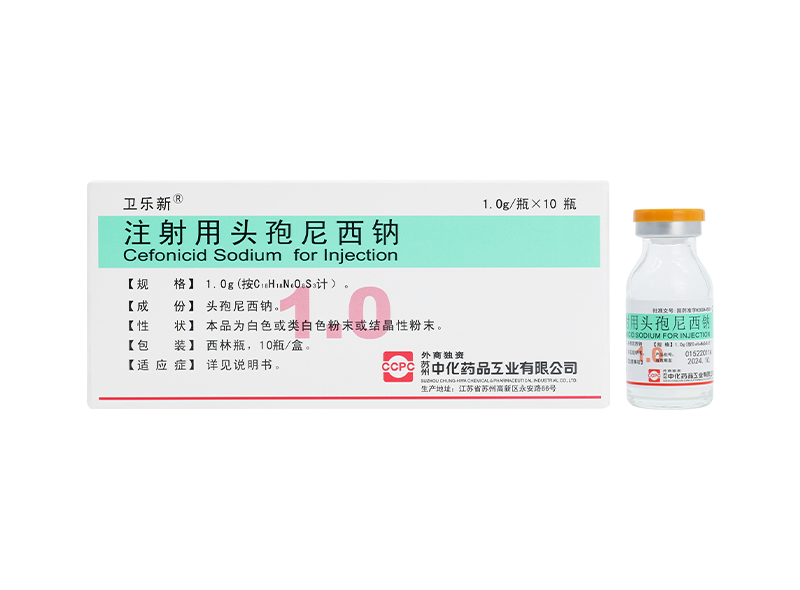 Weilexin® Cefonicid Sodium for Injection
Weilexin® Cefonicid Sodium for Injection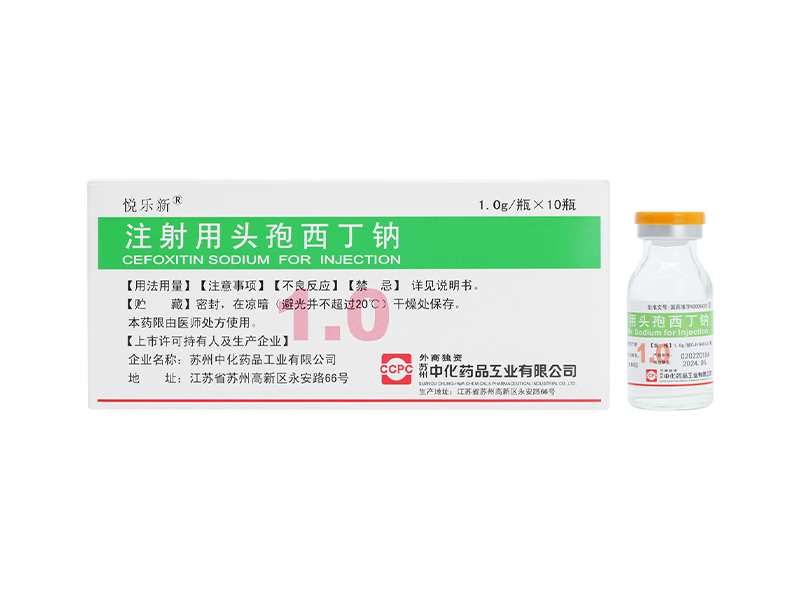 Yuelexin® Cefoxitin Sodium for Injection
Yuelexin® Cefoxitin Sodium for Injection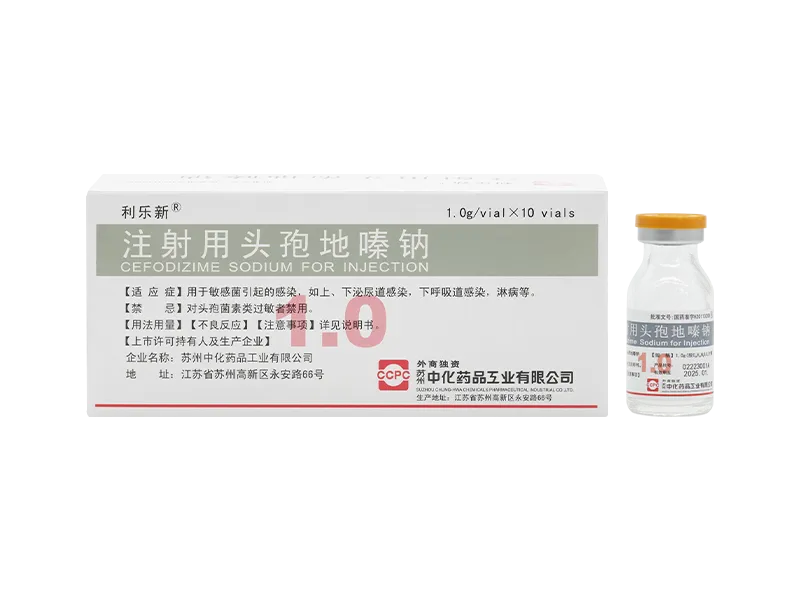 Lilexin® Cefodizime Sodium for Injection
Lilexin® Cefodizime Sodium for Injection Ailexin® Cefpirome Sulfate for Injection
Ailexin® Cefpirome Sulfate for Injection