- Home
- About Us
-
Products
-
 Fulexin® Cefuroxime Axetil Tablets
Fulexin® Cefuroxime Axetil Tablets -
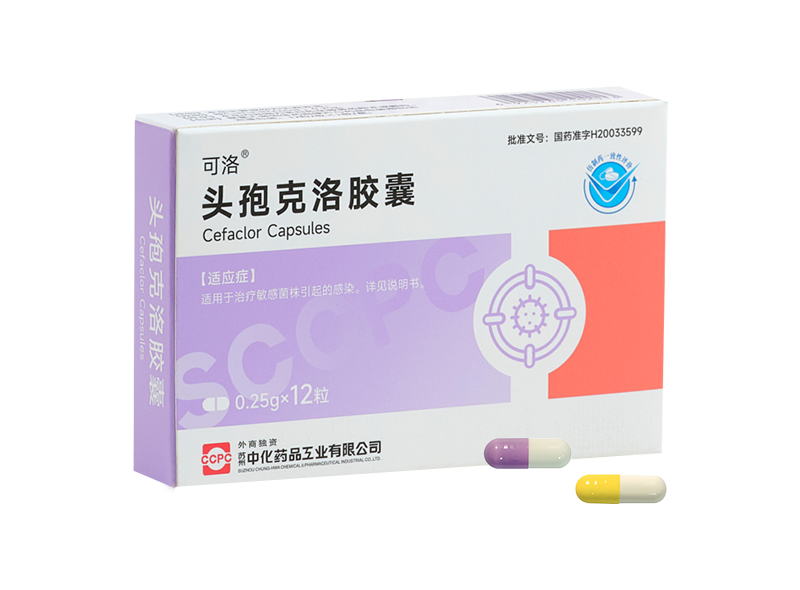 Keluo® Cefaclor Capsules
Keluo® Cefaclor Capsules -
 Kefu® Cefaclor Granules
Kefu® Cefaclor Granules -
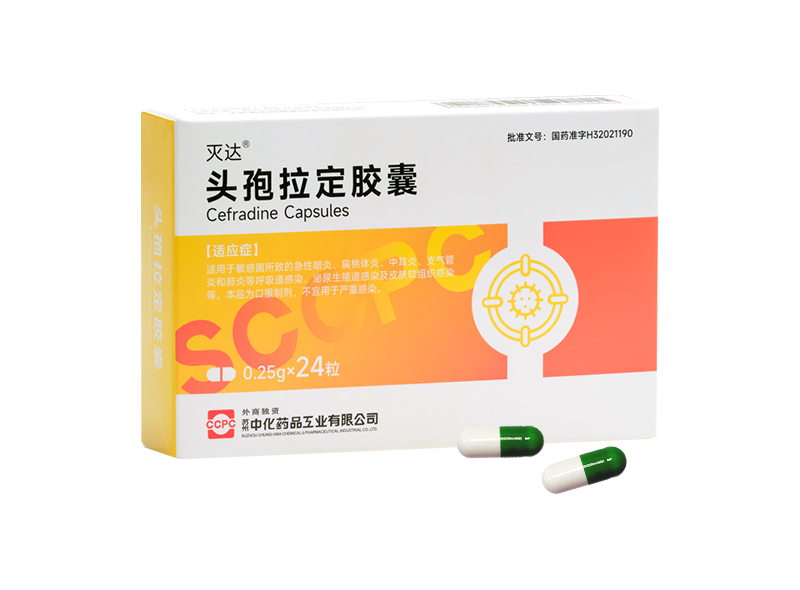 Mieda® Cefradine Capsules
Mieda® Cefradine Capsules -
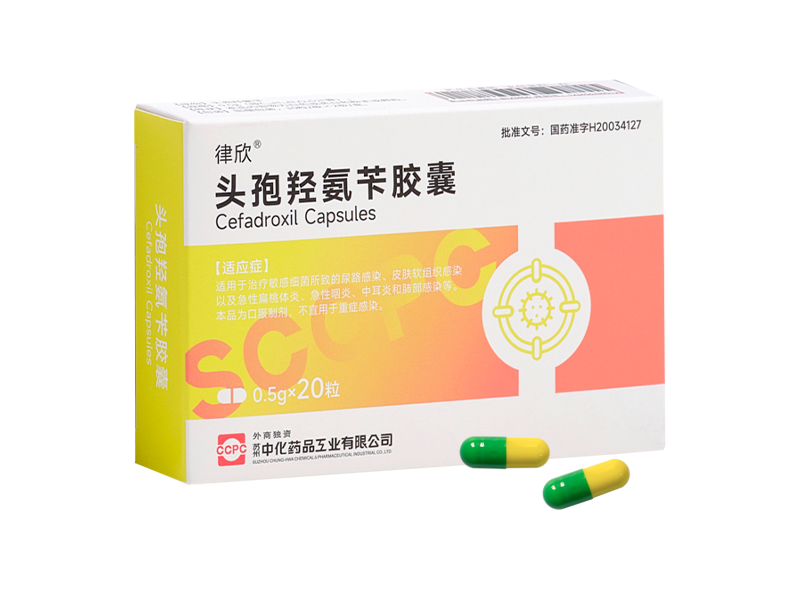 Lvxin® Cefalexin Capsules
Lvxin® Cefalexin Capsules -
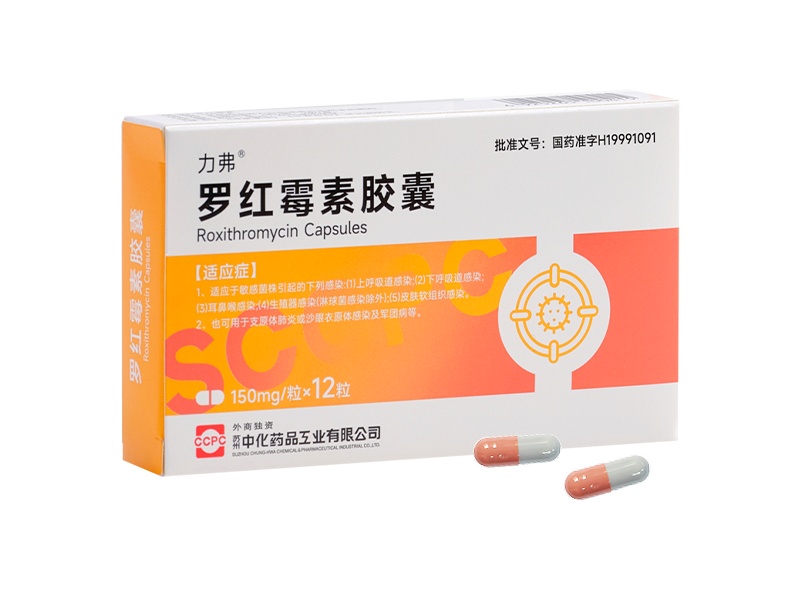 Lifu® Roxithromycin Capsules
Lifu® Roxithromycin Capsules -
 Lifu® Roxithromycin Granules
Lifu® Roxithromycin Granules -
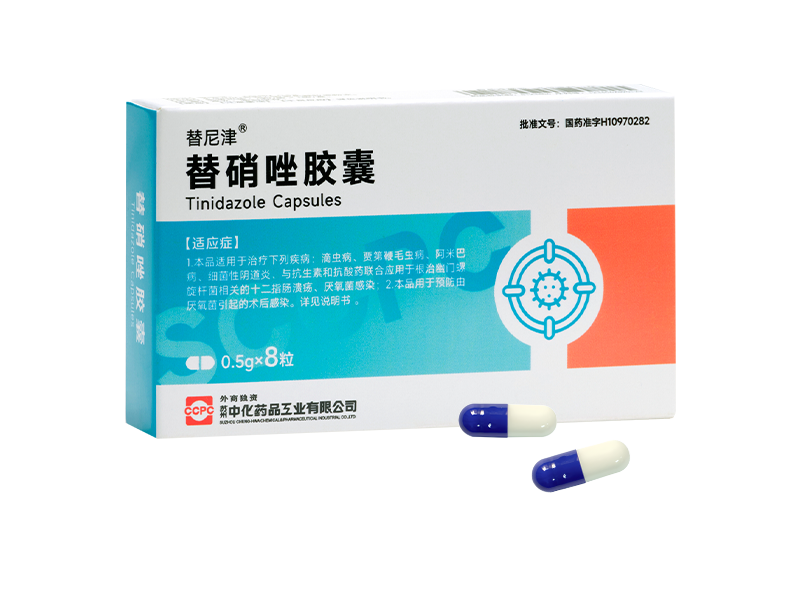 Tinijin® Tinidazole Capsules
Tinijin® Tinidazole Capsules -
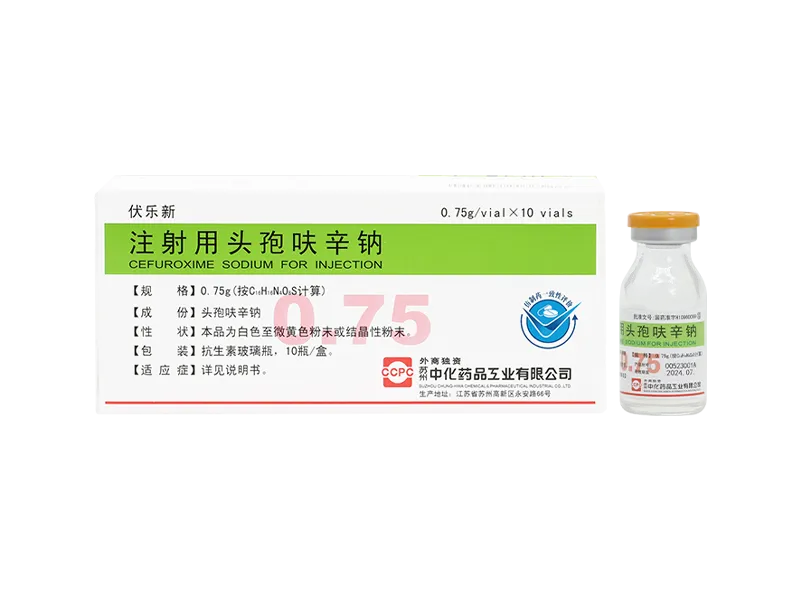 Fulexin® Cefazolin Sodium for Injection
Fulexin® Cefazolin Sodium for Injection -
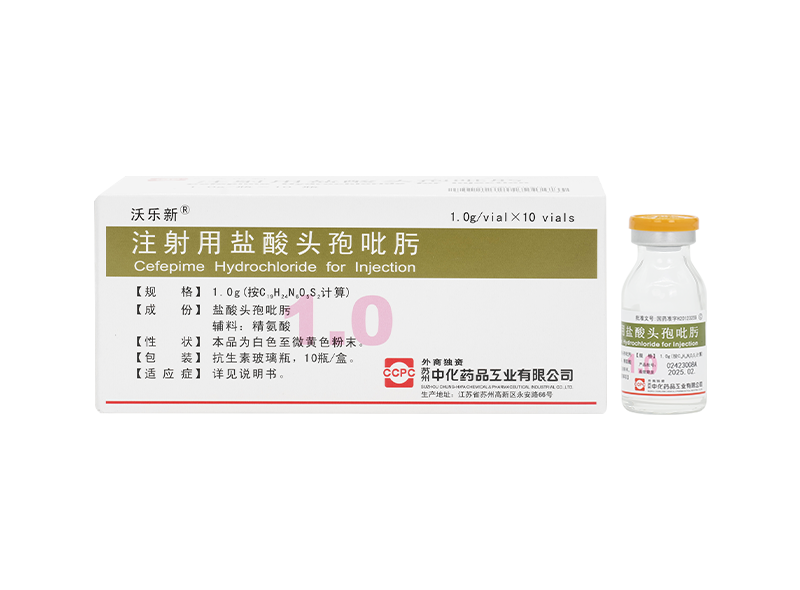 Wolexin® Cefepime Hydrochloride for Injection
Wolexin® Cefepime Hydrochloride for Injection -
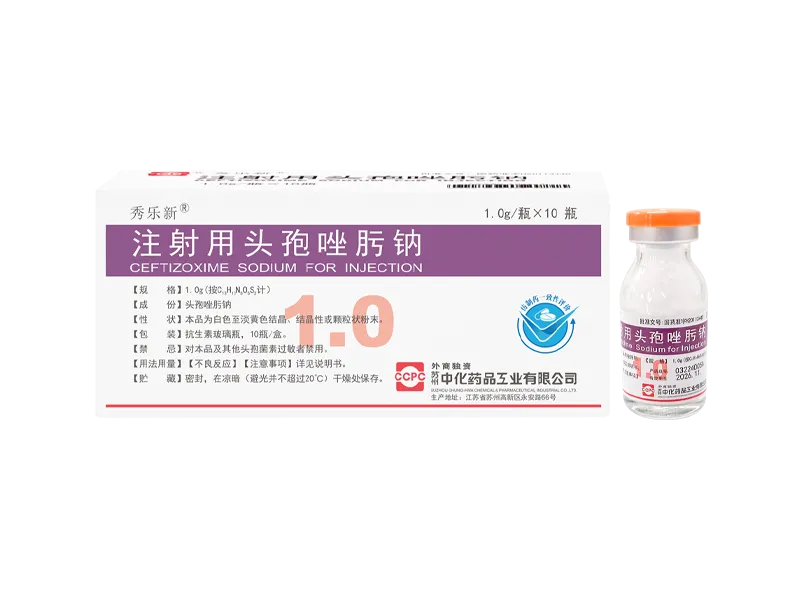 Xiulexin® Ceftizoxime Sodium for Injection
Xiulexin® Ceftizoxime Sodium for Injection -
 Xinlexin® Ceftriaxone Sodium for Injection
Xinlexin® Ceftriaxone Sodium for Injection -
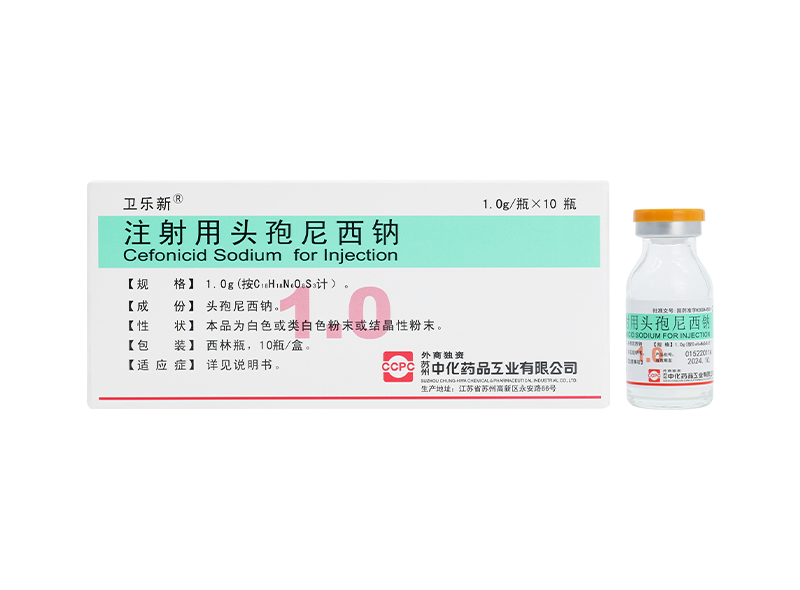 Weilexin® Cefonicid Sodium for Injection
Weilexin® Cefonicid Sodium for Injection -
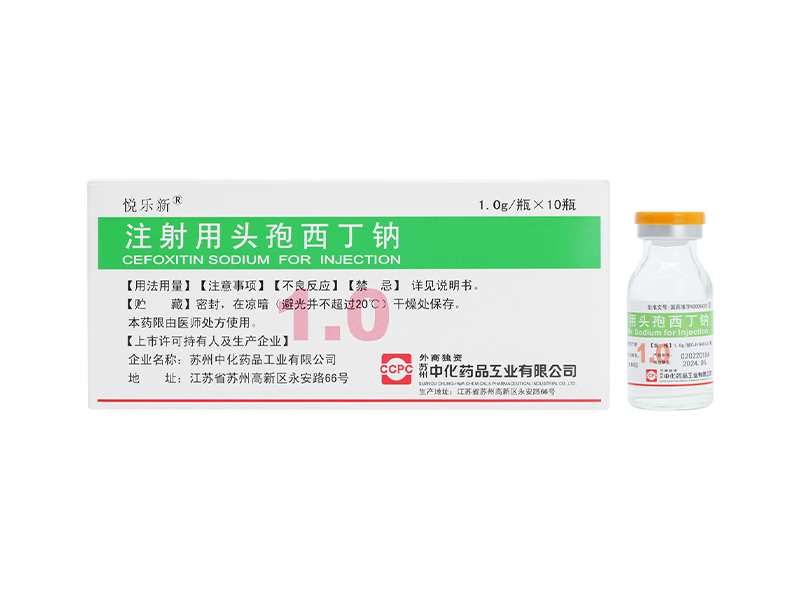 Yuelexin® Cefoxitin Sodium for Injection
Yuelexin® Cefoxitin Sodium for Injection -
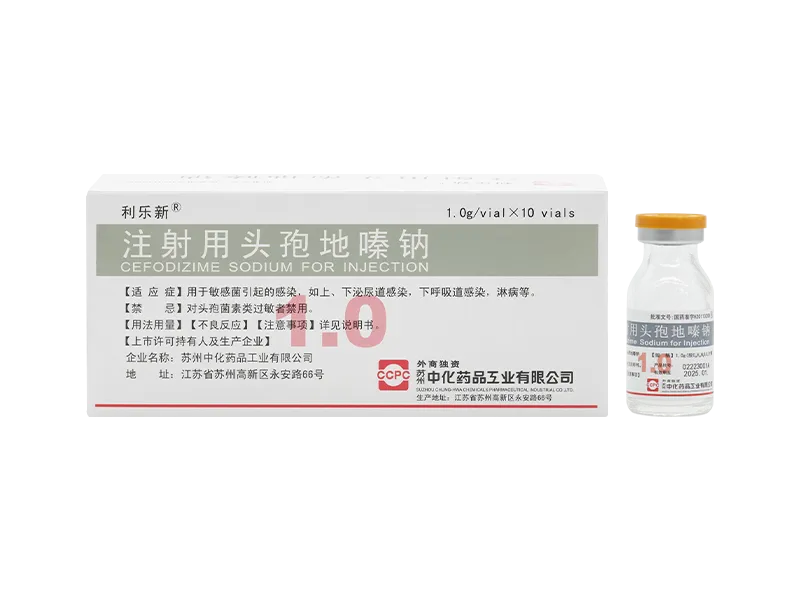 Lilexin® Cefodizime Sodium for Injection
Lilexin® Cefodizime Sodium for Injection -
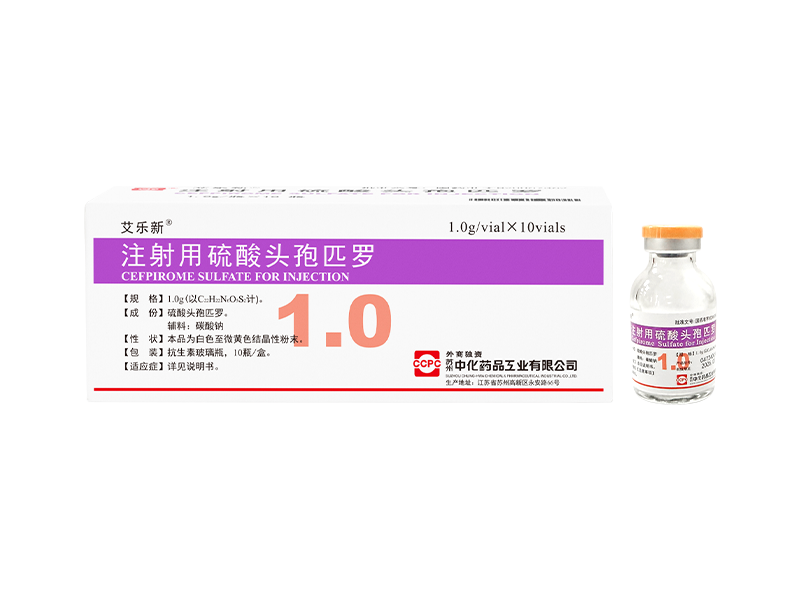 Ailexin® Cefpirome Sulfate for Injection
Ailexin® Cefpirome Sulfate for Injection
-
- Technology & Services
- CRO/CDMO
- Quality & Environmental Safety
- Contact Us