Suzhou Chung-Hwa Chemical & Pharmaceutical CDMO focuses on the research and production of small trial batches, pilot batches, validation batches and commercial batches of drugs. With more than 30 years of pharmaceutical enterprise background and accumulated pharmaceutical experience, it has formed the core advantages of excellent system, legal compliance, and combination of quality and cost, and has established a customer-centric and quality-based service principle, and is committed to providing customers with professional customized drug preparation R&D and production services.
Suzhou Chung-Hwa Chemical & Pharmaceutical has nearly 20 years of OEM experience at home and abroad, and has established cooperative relationships with more than 20 customers at home and abroad (commissioned approval, commissioned production and export), involving more than 40 specifications, covering China, Japan, Africa, the Philippines, Vietnam, Myanmar, Hong Kong and other countries and regions, and has been highly praised.
After passing the inspection and certification of PMDA of Japan in 2009, we officially started the road of customized R&D and production for Japanese pharmaceutical companies, which was well received by customers and continuously expanded the scope of business cooperation.
Since the introduction of the MAH system in 2019, we have successfully provided pilot batches, validation batches and commercial CDMO and CMO professional services to many well-known domestic companies, which have been fully recognized by customers.
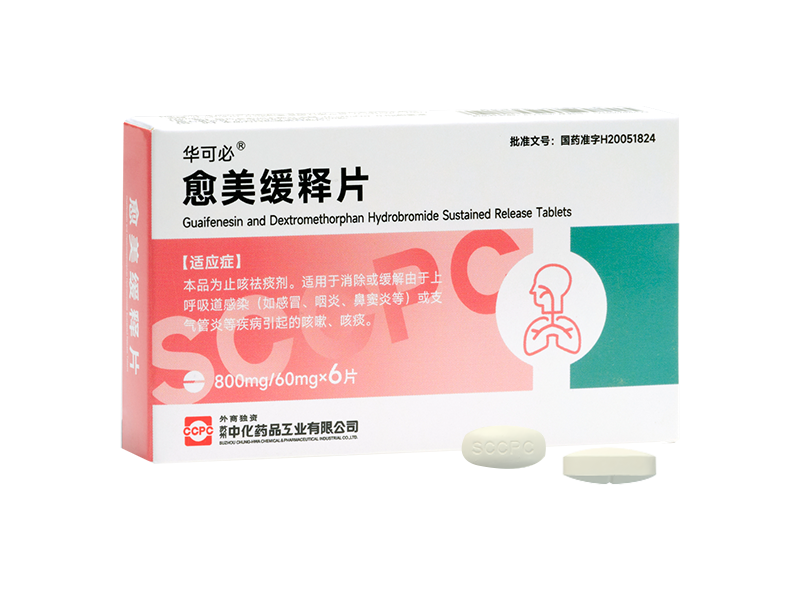
 Fulexin® Cefuroxime Axetil Tablets
Fulexin® Cefuroxime Axetil Tablets Keluo® Cefaclor Capsules
Keluo® Cefaclor Capsules Kefu® Cefaclor Granules
Kefu® Cefaclor Granules Mieda® Cefradine Capsules
Mieda® Cefradine Capsules Lvxin® Cefalexin Capsules
Lvxin® Cefalexin Capsules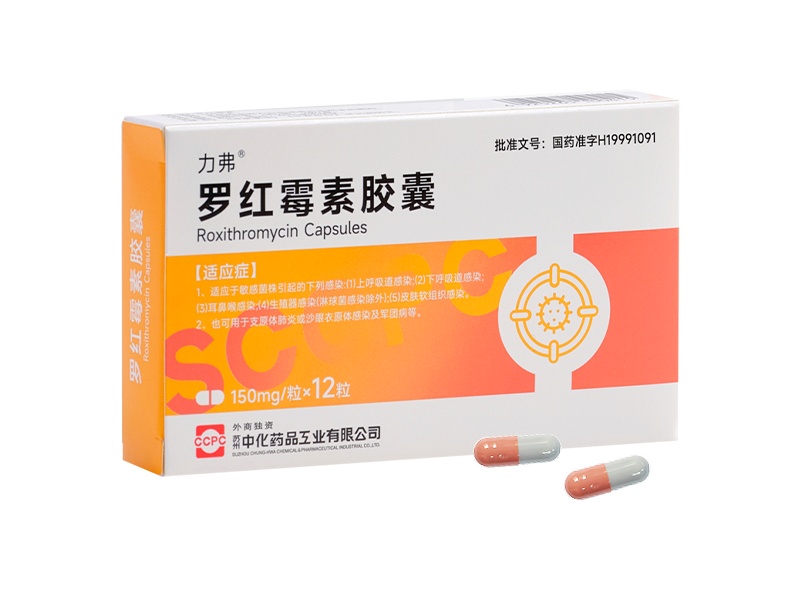 Lifu® Roxithromycin Capsules
Lifu® Roxithromycin Capsules Lifu® Roxithromycin Granules
Lifu® Roxithromycin Granules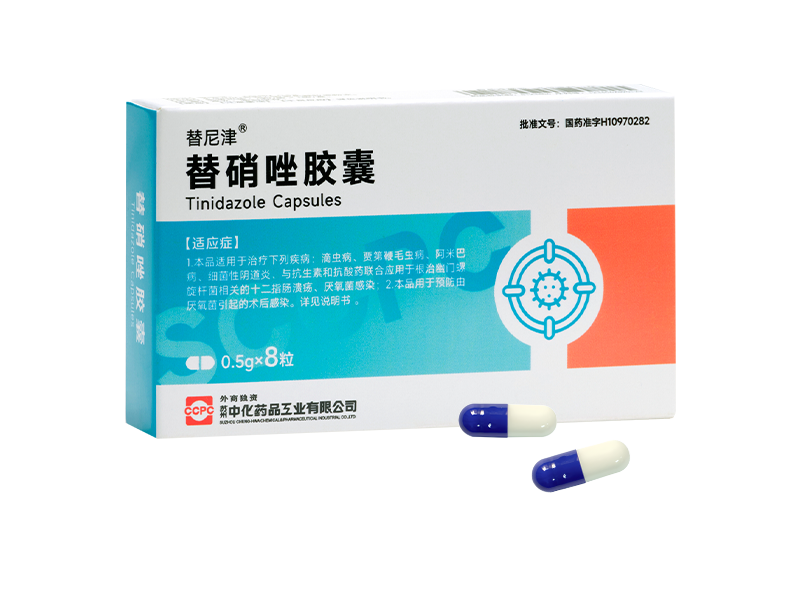 Tinijin® Tinidazole Capsules
Tinijin® Tinidazole Capsules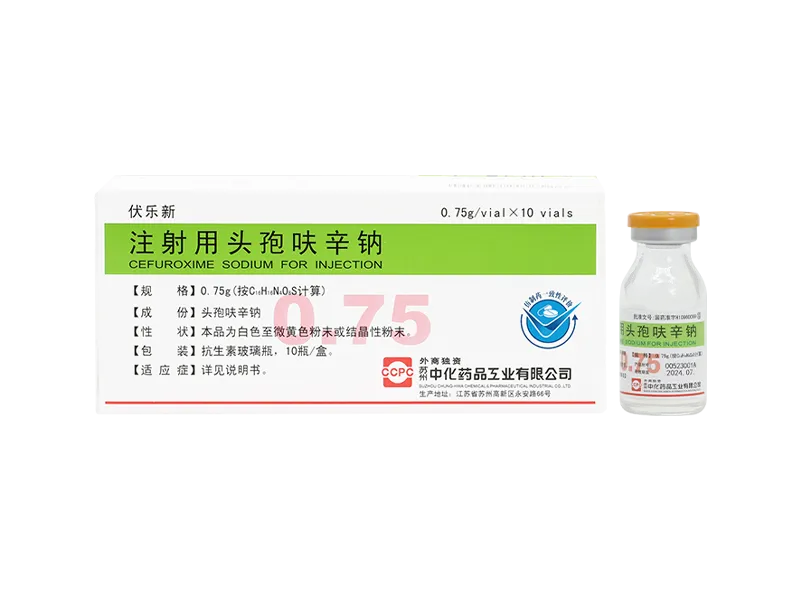 Fulexin® Cefazolin Sodium for Injection
Fulexin® Cefazolin Sodium for Injection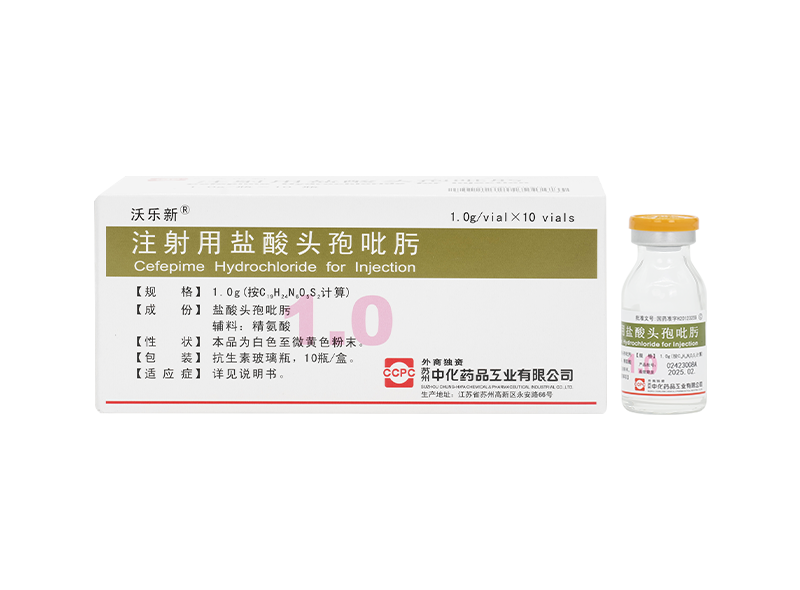 Wolexin® Cefepime Hydrochloride for Injection
Wolexin® Cefepime Hydrochloride for Injection Xiulexin® Ceftizoxime Sodium for Injection
Xiulexin® Ceftizoxime Sodium for Injection Xinlexin® Ceftriaxone Sodium for Injection
Xinlexin® Ceftriaxone Sodium for Injection Weilexin® Cefonicid Sodium for Injection
Weilexin® Cefonicid Sodium for Injection Yuelexin® Cefoxitin Sodium for Injection
Yuelexin® Cefoxitin Sodium for Injection Lilexin® Cefodizime Sodium for Injection
Lilexin® Cefodizime Sodium for Injection Ailexin® Cefpirome Sulfate for Injection
Ailexin® Cefpirome Sulfate for Injection