The 20th
Jiangsu Digestion & Digestive Endoscopy Progress Forum
From October 25 to 27, 2024, in the golden autumn, the "2024 Jiangsu Digestion & Digestive Endoscopy Progress Forum" hosted by the Jiangsu Anti-Cancer Association and organized by the Department of Gastroenterology and Digestive Endoscopy of Jiangsu Provincial People's Hospital (The First Affiliated Hospital of Nanjing Medical University, Jiangsu Provincial Maternal and Child Health Hospital) was held in the beautiful ancient capital of Nanjing, the Six Dynasties.

The 2024 Jiangsu Digestion & Digestive Endoscopy Progress Forum comprehensively showcased the latest research results in the field of digestive system and digestive endoscopy, and built an ideal platform for "publishing academic views and debating academic ideas". This conference combines academician forums, famous doctor lectures, academic speeches and operation demonstrations, and invites outstanding scholars and industry leaders from home and abroad to focus on academic topics such as endoscopic treatment of gastrointestinal fistulas, screening, diagnosis and treatment of early gastrointestinal cancer, new technologies in digestive endoscopy, gastrointestinal motility diseases, inflammatory bowel disease, portal hypertension, biliary and pancreatic diseases, Helicobacter pylori and gastrointestinal microecology, digestion & digestive endoscopy care. In addition, the forum also conducts standardized training, hands-on teaching and academic frontier presentations for gastroenterologists and surgeons at different levels and directions, releases the latest endoscopic guidelines, case discussions, learning center simulation operations and other academic exchange activities, providing an academic feast for the participants.
At the 2024 Jiangsu Digestion & Digestive Endoscopy Progress Forum, Professor Dai Fei from the Second Affiliated Hospital of Xi'an Jiaotong University introduced the definition and epidemiology of chronic constipation, progress in pathogenesis, progress in diagnostic methods and progress in treatment in the academic topic "Research Progress in Chronic Constipation", and listed commonly used therapeutic drugs, including bulk laxatives (calcium polycarbophil), osmotic laxatives, secretagogues, prokinetics, etc.

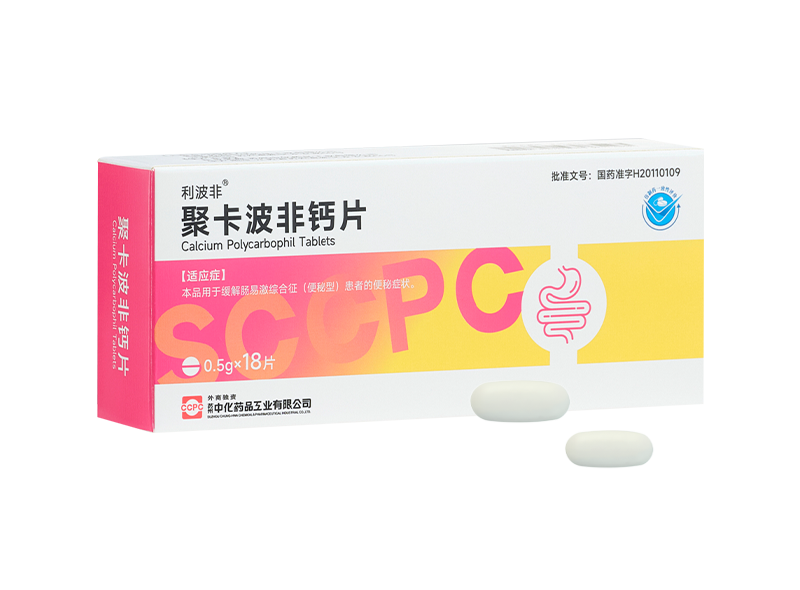
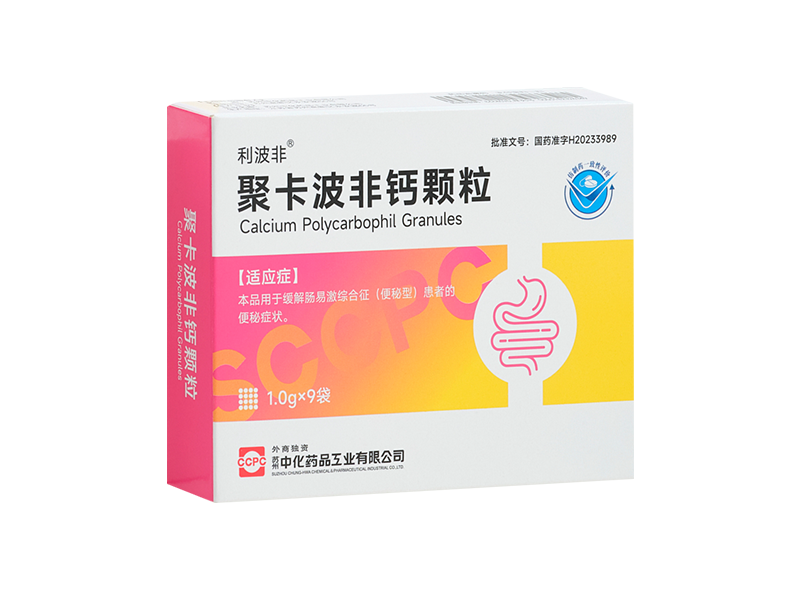
Well-known pharmaceutical company Suzhou Chung-Hwa Chemical & Pharmaceutical Industrial Co., Ltd. (hereinafter referred to as "Suzhou Chung-Hwa") participated in this Digestion & Digestive Endoscopy Progress Forum with its star product Libofei ® Calcium Polycarbophil Tablets/Granules, set up a beautiful booth, and distributed relevant daily practical information, patient education tools and exquisite gifts to the participants. Professional defecation management clinical guidelines and clinical application literature collection of calcium polycarbophil, Bristol score card, patient education manual, etc. can help doctors to have a deeper understanding of the clinical diagnosis and treatment dynamics related to constipation in daily life, facilitate doctors' clinical prescriptions, and improve the control of patients' treatment progress. The cute and healing cat image of the brand ambassador "Fei Pengpeng" attracted people to stop and was deeply loved by the participants.

Suzhou Chung-Hwa is a well-known pharmaceutical company with excellent R&D and production capabilities.As Suzhou Chung-Hwa's star product, Libofei ® calcium polycarbophil tablets/granules are clinically used to treat various types of constipation and have passed the national generic drug consistency evaluation. Its main ingredient is calcium polycarbophil, which is a bulk laxative and a basic drug for step-by-step treatment of constipation. It is also a first-line treatment drug clearly recommended by relevant domestic and foreign guidelines. After decalcification in the gastric acid environment, calcium polycarbophil can form a hydrophilic gel in the intestine, participate in the formation of feces, increase the water content and volume of feces, make the feces soft and easy to pass, and is not absorbed into the blood, so it is highly safe for clinical application.
Suzhou Chung-Hwa was established in April 1993. It uses the most advanced equipment and testing instruments, relies on the strength of Taiwan's China Chemical Pharmaceutical Research Institute, and integrates cross-strait research and development resources. It is a modern pharmaceutical company invested and established by Taiwan's China Chemical Pharmaceutical Co., Ltd. Since its establishment, Suzhou Chung-Hwa has won more than 50 annual awards. In 2020, it won the Advanced Enterprise Economic Contribution Award issued by the Hushuguan Economic and Technological Development Zone, and Chairman Wu Suhuan won two major awards for important contribution entrepreneurs. Over the years, it has been successively rated as a high-tech enterprise, a demonstration enterprise for the transformation of scientific and technological innovation results, and a responsible enterprise for paying tribute to the fight against the epidemic and ensuring supply.
 Fulexin® Cefuroxime Axetil Tablets
Fulexin® Cefuroxime Axetil Tablets Keluo® Cefaclor Capsules
Keluo® Cefaclor Capsules Kefu® Cefaclor Granules
Kefu® Cefaclor Granules Mieda® Cefradine Capsules
Mieda® Cefradine Capsules Lvxin® Cefalexin Capsules
Lvxin® Cefalexin Capsules Lifu® Roxithromycin Capsules
Lifu® Roxithromycin Capsules Lifu® Roxithromycin Granules
Lifu® Roxithromycin Granules Tinijin® Tinidazole Capsules
Tinijin® Tinidazole Capsules Fulexin® Cefazolin Sodium for Injection
Fulexin® Cefazolin Sodium for Injection Wolexin® Cefepime Hydrochloride for Injection
Wolexin® Cefepime Hydrochloride for Injection Xiulexin® Ceftizoxime Sodium for Injection
Xiulexin® Ceftizoxime Sodium for Injection Xinlexin® Ceftriaxone Sodium for Injection
Xinlexin® Ceftriaxone Sodium for Injection Weilexin® Cefonicid Sodium for Injection
Weilexin® Cefonicid Sodium for Injection Yuelexin® Cefoxitin Sodium for Injection
Yuelexin® Cefoxitin Sodium for Injection Lilexin® Cefodizime Sodium for Injection
Lilexin® Cefodizime Sodium for Injection Ailexin® Cefpirome Sulfate for Injection
Ailexin® Cefpirome Sulfate for Injection