01. Information and methods
1.1 General information
This study is a prospective, single-center, randomized controlled study. A total of 118 patients with IBS-C admitted to our hospital from January 2022 to January 2024 were selected. Inclusion criteria: (1) Age≥ 18 years; (2) Meet the diagnostic criteria for IBS-C as defined by the Rome IV criteria; (3) No morphological and structural changes were found during colonoscopy; Exclusion Criteria: (1) History of polycarbophil calcium and lactulose allergy; (2) Confirmed or suspected intestinal obstruction caused by tumors, hernias, etc.; (3) Severe abnormal liver function; (4) Difficulty
swallowing; (5) Suspected organic constipation; (6) Women who are pregnant, planning to become pregnant, and breastfeeding. The enrolled subjects were divided into control group and observation group according to the random number table method, with 59 cases each. There were no significant differences in gender, age, body mass index, and disease course between the two groups (P > 0.05).
1.2 Method
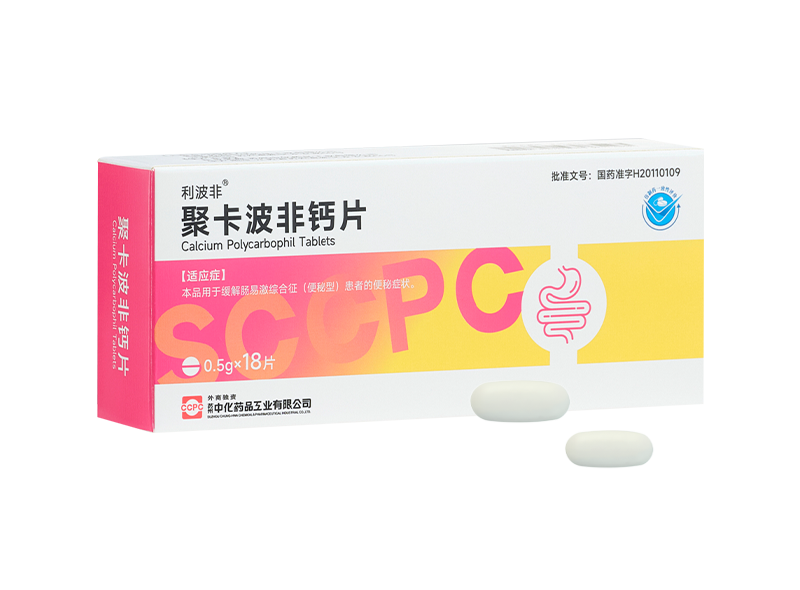
The control group was given lactulose oral solution, 30 minutes before breakfast, 30mL for the first time, then 15ml, 1 time/d, for 2 weeks. The observation group received polycarbofi calcium tablets (trade name: riprofil, Suzhou Sinochem Pharmaceutical Industry Co., Ltd., Sinopharm quasi-number: H20110109), 2 tablets each time, 0.5g /tablets, 3 times a day, for 2 weeks. No other laxative or interfering with gastrointestinal motility medications during treatment.
1.3 Observation indicators and evaluation criteria
The number of bowel movements is recorded by the patient or the patient's family in a bowel movement diary and reported to the healthcare returner, and the stool characteristics and consistency are assessed using the Bristol Stool Morphology Scale (BSFS). The Wexner Constipation Score (Chinese version) is used to assess constipation severity. According to the clinical symptom relief of patients, the clinical efficacy is divided into obvious, effective and ineffective. Obvious effect means that all or basic disappearance of the main symptoms, 5-9 bowel movements per week, stool forming, not hard, no mucus; It effectively represents the partial disappearance or reduction of the main symptoms, the number of bowel movements per week is 3-4 times, and the shape and frequency of stools have improved. Invalid means that the symptoms are still the same, the number of bowel movements per week is less than 3, and the shape and frequency of stool do not improve or the improvement is not obvious. Total clinical response rate = (number of effective people) / total number of cases ×100%. Peripheral blood samples of 5mL were taken on an empty stomach in the morning, left at room temperature for 30 minutes, and centrifuged to take the supernatant. Serum inflammatory factors interleukin (IL)-6, IL-8, and tumor necrosis factor-α (TNF-α) were determined by enzyme-linked immunosorbent assay (kits were purchased from Shanghai Yiyan Biotechnology Co., Ltd.).
The occurrence of adverse reactions during the treatment period of the two groups was recorded in detail.
1.4 Statistical analysis
SPSS 26.0 was used to statistically analyze the full-text data, and the normally distributed continuous data were expressed by mean ± standard deviation (Mean±SD), and the t-test was used for comparison between groups, and the paired t-test was used before and after treatment. Metrics that do not conform to the normal distribution are represented by median (interquartile range [IQR]), and the Wilcoxon signed-rank test is used to evaluate the change of index. Statistical data are expressed as frequency (n) and composition ratio (%), and comparisons between groups are statistically significant using Fisher's exact test, p < 0.05.
02. Results
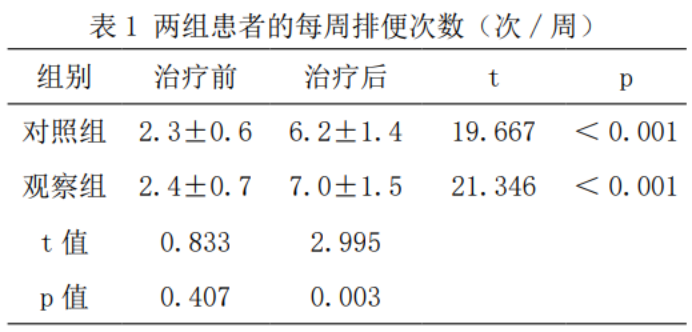
2.1 Number of bowel movements
Compared with before treatment, the weekly number of bowel movements in both groups increased significantly (p<0.05), and the average number of weekly bowel movements in the observation group was 7.0 times higher than that in the control group (t=2.995, p < 0.05), as shown in Table 1.
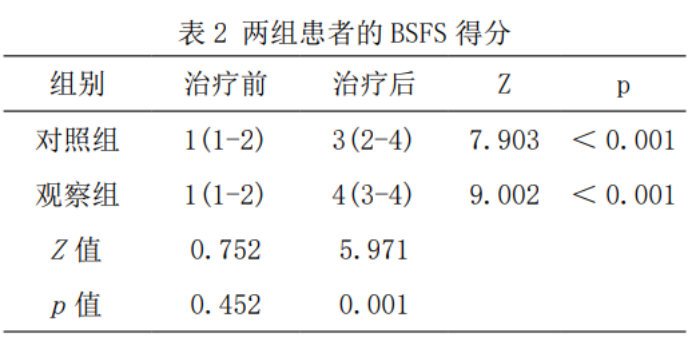
2.2 Fecal traits
Compared with before treatment, the BSFS scores of the two groups after treatment were significantly increased (p < 0.05). The BSFS score after treatment in the observation group was higher than that in the control group (Z=5.971, p < 0.05), as shown in Table 2.
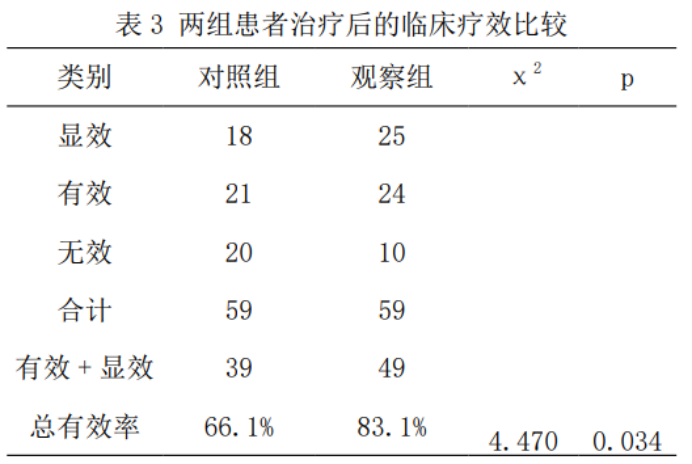
2.3 Comparison of clinical efficacy between the two groups
The overall effective rate of 83.1% in the observation group was significantly higher than that in the control group of 66.1% (χ2=4.470, p=0.034), as shown in Table 3.
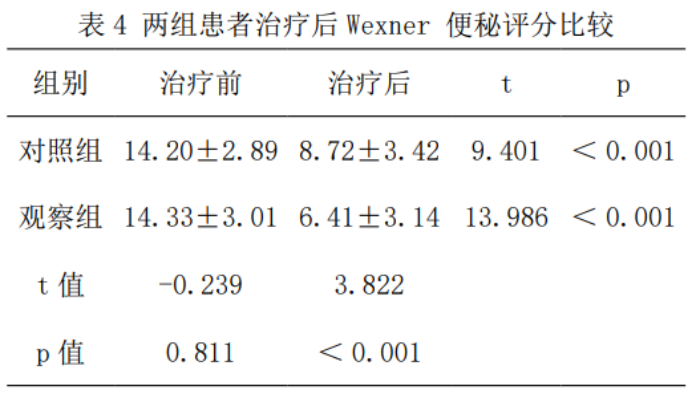
2.4 Degree of constipation
Compared with before treatment, the Wexner constipation score was significantly reduced in both groups after treatment (p < 0.001), and the Wexner constipation score after treatment was lower than that of the control group (t=3.822, p < 0.001), as shown in Table 4.
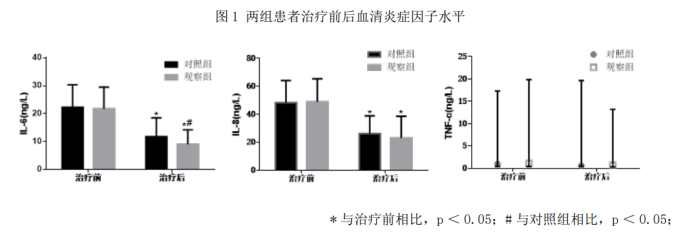
2.5 Relevant serum cytokine indicators
After treatment, the IL-6 significance in the observation group was lower than that in the control group (p< 0.05), and there was no statistically significant difference in TNF⁃α and IL-8 compared to the control group (p < 0.05), as shown in Figure 1.
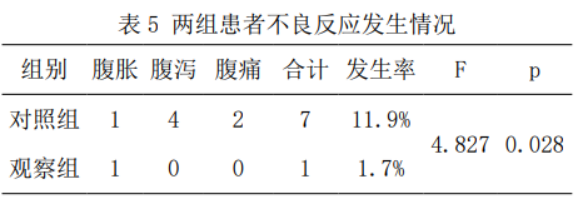
2.6 Adverse reactions
The incidence of adverse reactions in the control group was higher than that in the observation group, and the difference was statistically significant (11.9% vs. 1.7%, p=0.028), as shown in Table 5.
03. Discussion
IBS is a very common digestive tract disease, with a reported incidence of 1.4%-11.5% in our country. IBS-C is associated with higher bowel dysfunction such as abdominal pain and hard stools compared to other IBS subtypes. In addition, many patients experience other abdominal (discomfort, bloating) and intestinal-related symptoms(sparse, slaboring, incomplete bowel movements). Currently, there are many mechanisms of IBS-C, such as brain-gut axis dysfunction, inflammation, gut microbiome dysregulation, immune response, etc. IBS-C abdominal pain and less stool may be caused by changes in intestinal motility and water imbalance in the intestine. The clinical application of non-pharmacological treatment options for IBS-C is limited by disadvantages such as long time, economic cost, and insignificant effect. Osmotic laxatives and stimulant laxatives, although they can improve constipation symptoms, are mainly used for acute relief, and there is limited data from randomized controlled clinical trials in patients with IBS-C. Clinical studies on probiotics to improve IBS-C are still controversial due to low data quality, small sample sizes, strain types, and measurement tools. Long-term use of the 5-HT4 receptor agonists moxapride and cisapride can lead to increased cardiovascular events, and their efficacy in IBS-C has yet to be evaluated.
Polycarbofi calcium has extremely high water absorption properties, is not absorbed by the digestive tract, does not affect the activity of digestive enzymes, has sufficient stability and physiological inertness, and is often used to treat diarrhea and constipation symptoms of IBS. Although the therapeutic mechanism of IBS has not been elucidated, it has been reported that polycarbofi calcium can reduce hs-CRP levels in IBS-C, suggesting that polycarbofi calcium may alleviate IBS-C symptoms by reducing the level of microinflammation in the gut.
The incidence of adverse reactions between the two groups was 11.9% and 1.7%, respectively, with significant differences. Diarrhea and abdominal pain were the most common adverse reactions in the control group of this study, which is consistent with the results of phase III clinical studies and real-world studies that have been reported. In the randomized controlled experiments reported in China on the treatment of IBS-C patients, the most common adverse reactions were nausea, dry mouth, headache, dizziness and other mild symptoms, which gradually disappeared after discontinuation or continued medication. However, only one case of abdominal distension was observed in this study, and in short, polycarbophil calcium could reduce the overall incidence of adverse reactions compared with lactulose oral solution.
The serum levels of IL-6 and IL-8 in the observation and control groups were significantly reduced after treatment, and the levels of TNF-α did not change, suggesting that the level of inflammation in the two groups decreased after treatment. The pathophysiological mechanism of IBS-C is still unified, and more and more studies believe that intestinal mucosal inflammation is involved in the occurrence of IBS-C. A comparison with a control group of healthy normal people found that the serum levels of inflammatory factors IL-6 and IL-8 in IBS-C patients were significantly higher, indicating that elevated inflammatory factors were involved in the pathogenesis of IBS-C. The number of mast cells in the intestine of IBS-C patients increased significantly, and the large release of inflammatory mediators such as histamine induced hypersensitivity reactions in the intestines of IBS-C patients, causing abdominal pain. IL-6 levels were lower after combination than with IL-6 alone, suggesting that polycarbophil calcium may be involved in the process of IL-6 reduction. When inflammation occurs in the intestine, the intestinal mucosal barrier is disrupted, mucosal permeability increases, and pro-inflammatory factors such as IL-6 are released into the bloodstream in large quantities. There are still certain limitations in this study, firstly, this study is a single center and the sample size is small; Secondly, the results of this study may be affected by confounding factors such as diet and exercise. Finally, IBS-C is recurrent and requires long-term treatment and observation to evaluate medication regimens, and large-sample, long-period, multicenter real-world studies are needed in the future to evaluate the effect of polycarbophil calcium in patients with IBS-C.
In summary, the clinical effect of polycarbofecalcium in the treatment of IBS-C is remarkable, which can effectively increase the number of bowel movements, improve fecal characteristics, and relieve constipation, which is worthy of clinical promotion.
References :
[1] Li Bin, Qing Xianwei, Tang Le. Efficacy of polycarbophil calcium in the treatment of IBS-C and its effect on serum inflammatory factors[J]. Medical Innovation and Analysis, 2025, 3(3).
Scan the code to view the original text
 Fulexin® Cefuroxime Axetil Tablets
Fulexin® Cefuroxime Axetil Tablets Keluo® Cefaclor Capsules
Keluo® Cefaclor Capsules Kefu® Cefaclor Granules
Kefu® Cefaclor Granules Mieda® Cefradine Capsules
Mieda® Cefradine Capsules Lvxin® Cefalexin Capsules
Lvxin® Cefalexin Capsules Lifu® Roxithromycin Capsules
Lifu® Roxithromycin Capsules Lifu® Roxithromycin Granules
Lifu® Roxithromycin Granules Tinijin® Tinidazole Capsules
Tinijin® Tinidazole Capsules Fulexin® Cefazolin Sodium for Injection
Fulexin® Cefazolin Sodium for Injection Wolexin® Cefepime Hydrochloride for Injection
Wolexin® Cefepime Hydrochloride for Injection Xiulexin® Ceftizoxime Sodium for Injection
Xiulexin® Ceftizoxime Sodium for Injection Xinlexin® Ceftriaxone Sodium for Injection
Xinlexin® Ceftriaxone Sodium for Injection Weilexin® Cefonicid Sodium for Injection
Weilexin® Cefonicid Sodium for Injection Yuelexin® Cefoxitin Sodium for Injection
Yuelexin® Cefoxitin Sodium for Injection Lilexin® Cefodizime Sodium for Injection
Lilexin® Cefodizime Sodium for Injection Ailexin® Cefpirome Sulfate for Injection
Ailexin® Cefpirome Sulfate for Injection