On November 12, 2024, our company received the "Notice of Approval of Drug Supplementary Application" for Cefuroxime Sodium for Injection (Notice No.: 2024B05171, 2024B05175) approved and issued by the National Drug Administration, which means that our company's Cefuroxime Sodium for Injection 0.5g and 1.0g dual specifications have passed the national generic drug quality and efficacy consistency evaluation.

Cefuroxime sodium belongs to the third generation of cephalosporin antibiotics, which has the characteristics of low resistance, broad antibacterial spectrum and high safety. It is mainly used to treat lower respiratory tract infections, urinary tract infections, abdominal infections, pelvic infections, sepsis, skin and soft tissue infections, bone and joint infections, meningitis and simple gonorrhea caused by sensitive bacteria. Suzhou Chung-Hwa's Cefuroxime sodium for injection was launched in 1996 and has been widely recognized by doctors and patients for its reliable clinical efficacy and safety for more than 20 years.
Suzhou Chung-Hwa has always been adhering to the core values of "passion, innovation, care, and health", focusing on quality, insisting on continuous innovation, constantly improving core competitiveness, and continuously committed to providing more safe, effective, quality-controlled, clinically accessible treatment options and health services for patients. So far, the company has a total of 11 varieties and 20 specifications that have passed the consistency evaluation.

The anti-infection field is a key advantage area for Suzhou Chung-Hwa, and the company has a series of cephalosporin products from the first to the fourth generation. The approval of the dual specifications of Cefuroxime Sodium for Injection also means that Suzhou Chung-Hwa's anti-infection product line has been further expanded and improved, and will provide more drug options for anti-infection treatment in the future.

 Fulexin® Cefuroxime Axetil Tablets
Fulexin® Cefuroxime Axetil Tablets Keluo® Cefaclor Capsules
Keluo® Cefaclor Capsules Kefu® Cefaclor Granules
Kefu® Cefaclor Granules Mieda® Cefradine Capsules
Mieda® Cefradine Capsules Lvxin® Cefalexin Capsules
Lvxin® Cefalexin Capsules Lifu® Roxithromycin Capsules
Lifu® Roxithromycin Capsules Lifu® Roxithromycin Granules
Lifu® Roxithromycin Granules Tinijin® Tinidazole Capsules
Tinijin® Tinidazole Capsules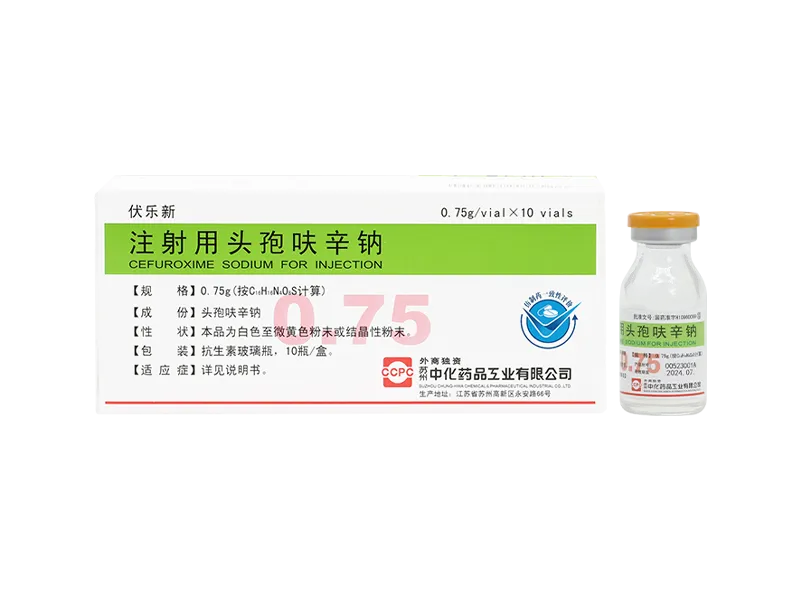 Fulexin® Cefazolin Sodium for Injection
Fulexin® Cefazolin Sodium for Injection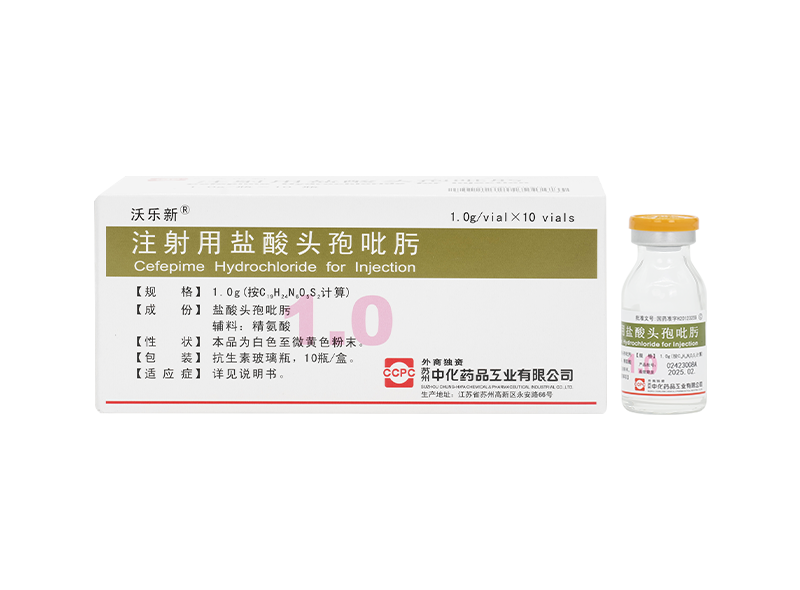 Wolexin® Cefepime Hydrochloride for Injection
Wolexin® Cefepime Hydrochloride for Injection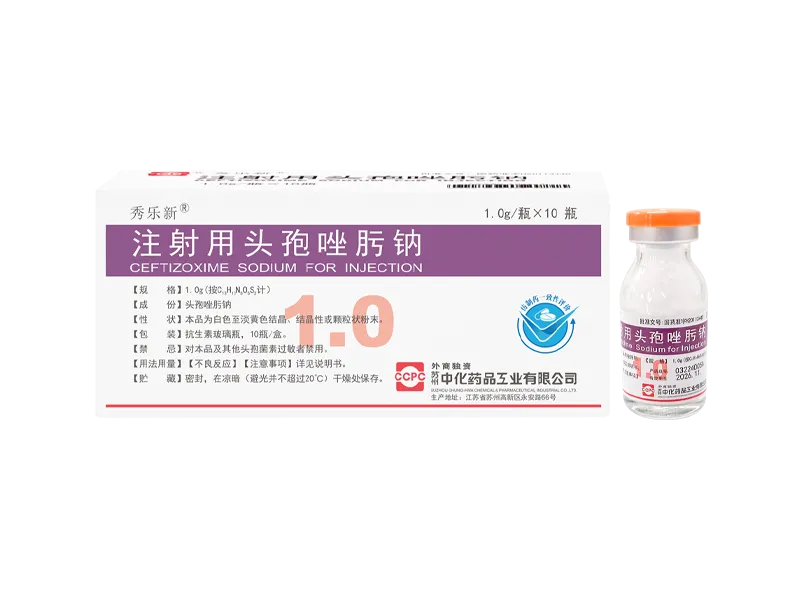 Xiulexin® Ceftizoxime Sodium for Injection
Xiulexin® Ceftizoxime Sodium for Injection Xinlexin® Ceftriaxone Sodium for Injection
Xinlexin® Ceftriaxone Sodium for Injection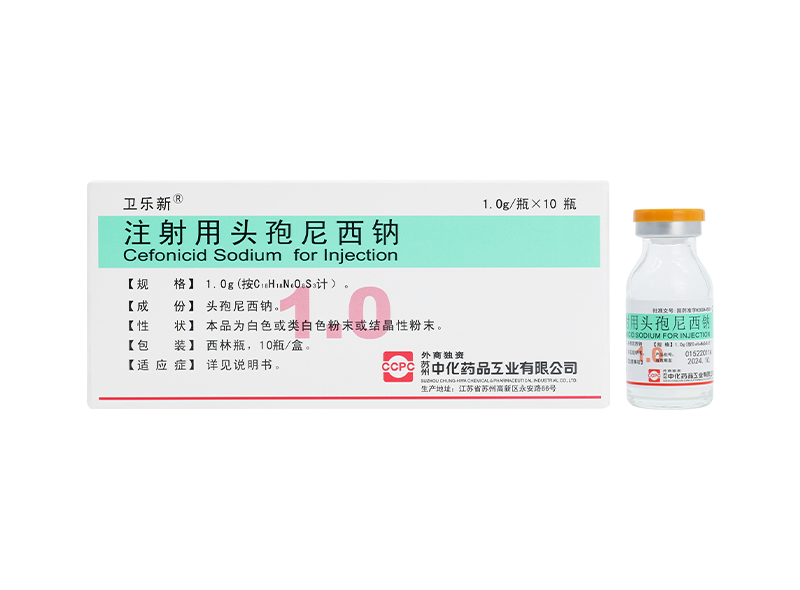 Weilexin® Cefonicid Sodium for Injection
Weilexin® Cefonicid Sodium for Injection Yuelexin® Cefoxitin Sodium for Injection
Yuelexin® Cefoxitin Sodium for Injection Lilexin® Cefodizime Sodium for Injection
Lilexin® Cefodizime Sodium for Injection Ailexin® Cefpirome Sulfate for Injection
Ailexin® Cefpirome Sulfate for Injection