Recently, Suzhou Chung-Hwa Chemical & Pharmaceutical participated in the "12th Cross-Strait Digestive Forum and World Chinese Digestive Innovation Forum, the 7th World Chinese Digestive Youth Forum, 2024 Chongqing Digestive and Digestive Endoscopy Academic Annual Conference" co-organized by Chongqing Medical Association, Chongqing Medical Association Gastroenterology and Digestive Endoscopy Branch, Cross-Strait Peaceful Development Collaborative Innovation Center, Taiwan Digestive Medical Association, Digestive Endoscopy Medical Association, Hong Kong Digestive Society, Digestive Endoscopy Society, and co-organized by the Second Affiliated Hospital of Chongqing Medical University, Xiamen University Affiliated Zhongshan Hospital, Prince of Wales Hospital of the Chinese University of Hong Kong, Hospital Affiliated to National Taiwan University School of Medicine, Linkou Chang Gung Memorial Hospital of Chang Gung University, Taiwan, and Kaohsiung Medical University Affiliated Hospital. The conference was held in Chongqing from November 29 to December 1, 2024 and has been successfully concluded.

This conference invited authoritative experts of Chinese descent from mainland China, Taiwan, Hong Kong, Macao and overseas to conduct multidisciplinary, multi-angle and in-depth exchanges, jointly explore innovative strategies under the current situation and reach consensus, laying the foundation for further improving the diagnosis and treatment of digestive system diseases on both sides of the Taiwan Strait and in Hong Kong and Macao.

▲Professor Chiu Cheng-tang, Chairman of the Taiwan Society of Digestive Endoscopy, delivered a speech

▲Executive Director of Cross-Strait Medical and Health Exchange Association
Professor Ren Jianlin, Chairman of the Gastroenterology Branch, delivered a speech

▲Professor Liu Runhuang of Prince of Wales Hospital, The Chinese University of Hong Kong, delivering a speech
Suzhou Chung-Hwa Chemical & Pharmaceutical was invited to participate in this event and set up an exclusive booth area with its star product Libofei ® Calcium Polycarbophil Tablets/Granules. In this event, the brand image spokesperson "Fei Pengpeng" became the focus of the event with his professional, cute and healing cat image. In addition, the booth set up a number of interesting interactive sessions, which promoted the interaction and communication between the participants and Suzhou Chung-Hwa Chemical & Pharmaceutical, and also allowed the participants to more intuitively understand the advantages and characteristics of Libofei ® Calcium Polycarbophil Tablets/Granules.

According to the on-site staff, Libofei® calcium polycarbophil tablets/granules are well-known products of Suzhou Chung-Hwa Chemical & Pharmaceutical. They are bulking laxatives, also known as stool bulking agents, and are clinically used to treat various types of constipation, such as constipation caused by irritable bowel syndrome, functional constipation, constipation caused by benign anal diseases, constipation in postoperative patients, etc. This product has passed the national generic drug consistency evaluation, bringing a safe and effective new option for constipation treatment in the clinic, and providing a new treatment plan for clinicians to treat constipation.

At the same time, Libofei® calcium polycarbophil tablets/granules, as a representative bulk-forming laxative, have been recommended by multiple expert organizations. The "WGO Global Guidelines: Constipation" recommends graded diagnosis and treatment of constipation. The first step is to improve lifestyle and take fiber supplements or other bulk-forming laxatives; the "American Gastroenterological Association Medical Position Review: Constipation" also recommends soluble fiber or supplements, such as calcium polycarbophil.

About Suzhou Chung-Hwa Chemical & Pharmaceutical
Suzhou Chung-Hwa Chemical & Pharmaceutical is the manufacturer of Libofei ® . As a well-known pharmaceutical company, it has excellent R&D and production capabilities. It is one of the earliest pharmaceutical companies in China to pass GMP certification. It uses the most advanced equipment and testing instruments, relies on the strength of Taiwan's China Chemical Pharmaceutical Research Institute, and integrates cross-strait R&D resources. The main drug categories currently include: digestive system drugs, cardiovascular system drugs, skeletal muscle system drugs, endocrine system drugs, anti-infective drugs, etc. Since its establishment, Suzhou Chung-Hwa Chemical & Pharmaceutical has won more than 50 annual awards. In 2020, it won the Advanced Enterprise Economic Contribution Award issued by the Hushuguan Economic and Technological Development Zone, and Chairman Wu Suhuan won two awards for important contribution entrepreneurs. Over the years, it has been successively rated as a high-tech enterprise, a demonstration enterprise for the transformation of scientific and technological innovation results, and a responsible enterprise for paying tribute to the fight against the epidemic and ensuring supply.
The 12th Cross-Strait Digestive Disease Forum promoted academic exchanges in the fields of digestive diseases, digestive endoscopy, and liver diseases across the Taiwan Straits, and enhanced mutual understanding between professional physicians on both sides of the Taiwan Straits. Suzhou Chung-Hwa Chemical & Pharmaceutical participated in this event and conducted in-depth exchanges with academia and the industry, mastering the cutting-edge information and development trends of the industry. In the future, Suzhou Chung-Hwa Chemical & Pharmaceutical will continue to uphold the core values of "passion, innovation, care, and health" and contribute to the health cause of the Chinese world.
 Fulexin® Cefuroxime Axetil Tablets
Fulexin® Cefuroxime Axetil Tablets Keluo® Cefaclor Capsules
Keluo® Cefaclor Capsules Kefu® Cefaclor Granules
Kefu® Cefaclor Granules Mieda® Cefradine Capsules
Mieda® Cefradine Capsules Lvxin® Cefalexin Capsules
Lvxin® Cefalexin Capsules Lifu® Roxithromycin Capsules
Lifu® Roxithromycin Capsules Lifu® Roxithromycin Granules
Lifu® Roxithromycin Granules Tinijin® Tinidazole Capsules
Tinijin® Tinidazole Capsules Fulexin® Cefazolin Sodium for Injection
Fulexin® Cefazolin Sodium for Injection Wolexin® Cefepime Hydrochloride for Injection
Wolexin® Cefepime Hydrochloride for Injection Xiulexin® Ceftizoxime Sodium for Injection
Xiulexin® Ceftizoxime Sodium for Injection Xinlexin® Ceftriaxone Sodium for Injection
Xinlexin® Ceftriaxone Sodium for Injection Weilexin® Cefonicid Sodium for Injection
Weilexin® Cefonicid Sodium for Injection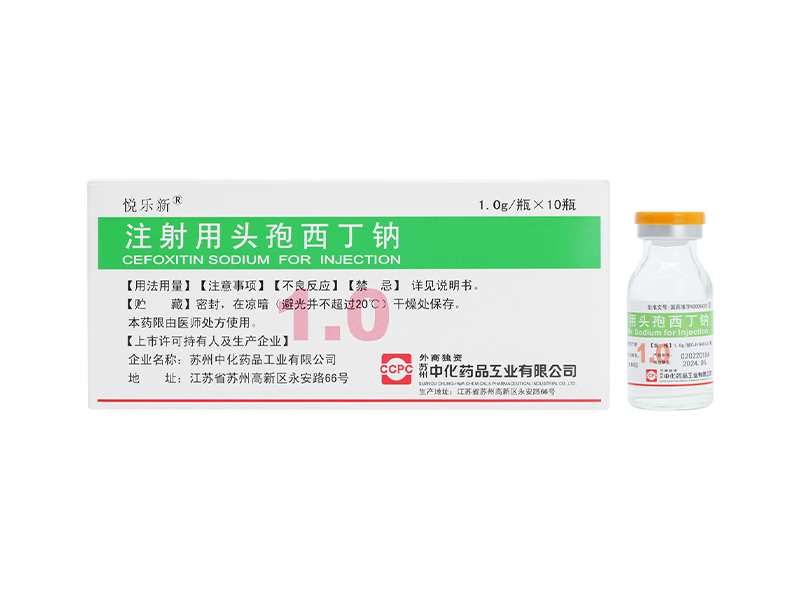 Yuelexin® Cefoxitin Sodium for Injection
Yuelexin® Cefoxitin Sodium for Injection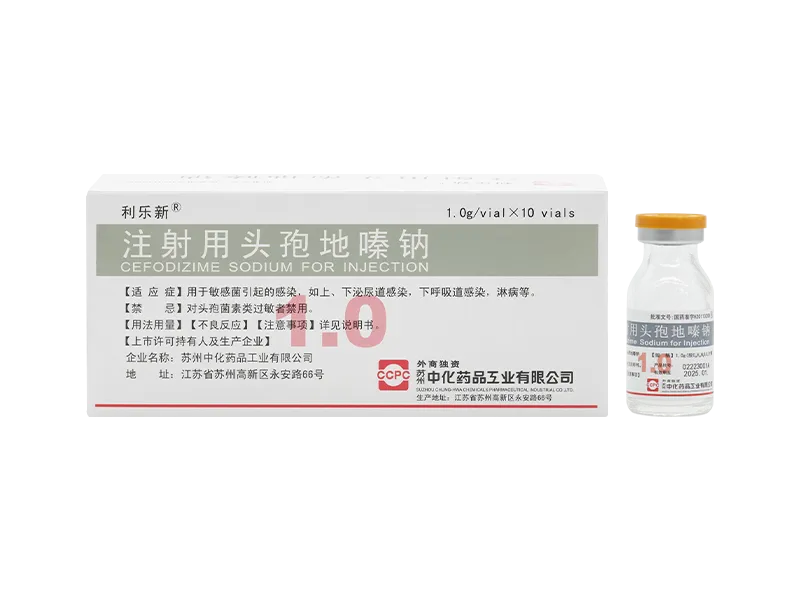 Lilexin® Cefodizime Sodium for Injection
Lilexin® Cefodizime Sodium for Injection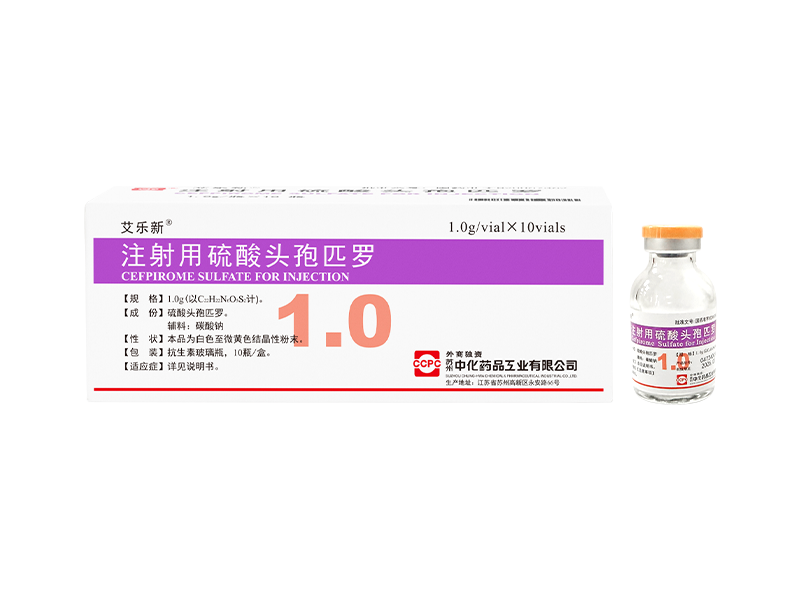 Ailexin® Cefpirome Sulfate for Injection
Ailexin® Cefpirome Sulfate for Injection