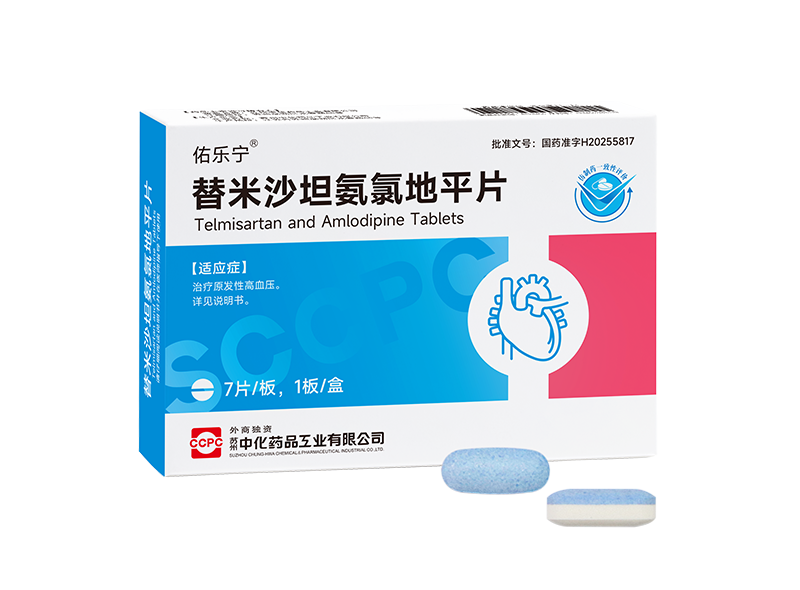
Recently, three new products from Suzhou Sinochem Pharmaceuticals—Tongbang® Glucosamine Sulfate Capsules, Youlening® (Telmisartan-Amlodipine Tablets 80mg/5mg), and Hualoning® Theophylline Extended-Release Tablets—have successively been approved for marketing by the National Medical Products Administration. The rapid approval of these three new products not only brings more clinical treatment options for patients in the fields of musculoskeletal and cardiovascular diseases, but also demonstrates Suzhou Sinochem Pharmaceuticals' continuously accelerating R&D efficiency and steadily growing comprehensive strength.
Multiple breakthroughs in core areas
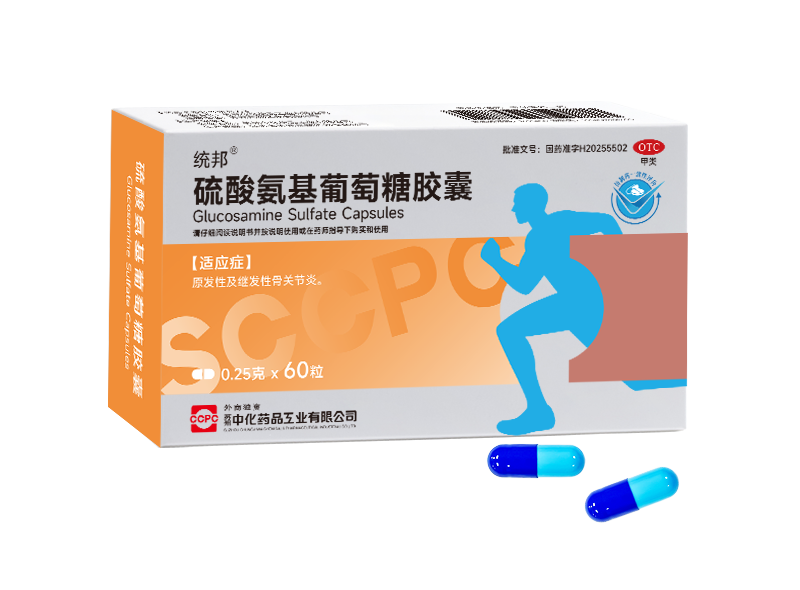
A new powerhouse for musculoskeletal health: Tongbang® Glucosamine Sulfate Capsules
Ammonium sulfate glucosamine capsules, as a 'joint cartilage protector,' can improve joint pain and function, and are suitable for the treatment of primary and secondary osteoarthritis. They aim to repair cartilage, strengthen joints, and promote the repair and reconstruction of cartilage matrix, thereby potentially slowing the pathological process of osteoarthritis pain and disease progression, improving joint mobility, relieving pain, and enhancing quality of life.
Cardiovascular Pipeline Shines with Two Stars: Youlening® Telmisartan and Amlodipine Tablets, Hualoning® Hexo Ketococoa Sustained-Release Tablets
Youle Ning® Telmisartan Amlodipine Tablets: Treating hypertension often requires a synergistic effect through multiple mechanisms and targets. Youle Ning® is a single-pill combination of two first-line classic antihypertensive drugs — the angiotensin II receptor blocker (ARB) telmisartan and the long-acting dihydropyridine calcium channel blocker (CCB) amlodipine. These two ingredients work through complementary mechanisms to achieve a synergistic effect while counteracting each other's side effects.
Hualoning® Propentofylline Sustained-Release Tablets: As a nonspecific phosphodiesterase inhibitor, propentofylline possesses multiple pharmacological effects, including enhancing red blood cell deformability, inhibiting platelet aggregation, dilating blood vessels, and anti-inflammatory actions, and has been included in the national medical insurance Class B catalog. This drug is mainly used to treat peripheral vascular diseases, such as symptoms of intermittent claudication caused by chronic peripheral arterial occlusive disease, and it can also be used to improve cerebral circulation following ischemic cerebrovascular disease.
Systematic Innovation-Driven
The successful approval of three new drugs demonstrates Suzhou SinoPharma's more than thirty years of
accumulated strength and its efficient integration within the 'R&D-production-quality control' excellence system.
Forward-looking and efficient R&D-driven approach: The company consistently adheres to a clinical demand-oriented strategy, emphasizing the deployment of new products and investment in research and development. Through scientific project management and efficient R&D processes, it ensures seamless coordination from project initiation to regulatory submission, significantly improving R&D efficiency and enabling rapid translation of innovative results.
Lean and intelligent manufacturing: Leveraging production bases that meet international standards and advanced manufacturing processes, the company ensures the stability and reliability of every medication. Intelligent and automated production lines provide a solid guarantee for high-quality, large-scale production of products.
Stringent and consistent quality control system: From raw material procurement to product release, Suzhou Zhonghua Pharmaceuticals has established a comprehensive quality management system covering the entire process (GMP). By strictly following pharmaceutical production quality management standards, it ensures that every pill delivered to patients is safe, effective, and of outstanding quality.
This systematic capability provides core assurance for the efficient and high-quality translation of innovative results from the laboratory to the market, and actively demonstrates its commitment to patient safety.
Suzhou Sinochem Pharmaceuticals will continue to uphold the vision of being a “trusted healthcare enterprise in the Chinese-speaking world,” adhere to the core values of “innovation, health, passion, and care,” and maintain a strong focus on multiple key areas such as endocrinology, gastroenterology, cardiovascular, and central nervous system. The company is committed to providing patients with multidimensional, full-cycle health solutions. The simultaneous approval of three new drugs not only demonstrates the company’s solid strength in research and innovation but also represents an important initiative in actively supporting the “Healthy China” strategy. In the future, Suzhou Sinochem Pharmaceuticals will continue to uphold a rigorous scientific approach and high-quality standards, continuously promoting the introduction of more high-quality medicines and healthcare services, providing more comprehensive and reliable health protection for Chinese people worldwide.
 Fulexin® Cefuroxime Axetil Tablets
Fulexin® Cefuroxime Axetil Tablets Keluo® Cefaclor Capsules
Keluo® Cefaclor Capsules Kefu® Cefaclor Granules
Kefu® Cefaclor Granules Mieda® Cefradine Capsules
Mieda® Cefradine Capsules Lvxin® Cefalexin Capsules
Lvxin® Cefalexin Capsules Lifu® Roxithromycin Capsules
Lifu® Roxithromycin Capsules Lifu® Roxithromycin Granules
Lifu® Roxithromycin Granules Tinijin® Tinidazole Capsules
Tinijin® Tinidazole Capsules Fulexin® Cefazolin Sodium for Injection
Fulexin® Cefazolin Sodium for Injection Wolexin® Cefepime Hydrochloride for Injection
Wolexin® Cefepime Hydrochloride for Injection Xiulexin® Ceftizoxime Sodium for Injection
Xiulexin® Ceftizoxime Sodium for Injection Xinlexin® Ceftriaxone Sodium for Injection
Xinlexin® Ceftriaxone Sodium for Injection Weilexin® Cefonicid Sodium for Injection
Weilexin® Cefonicid Sodium for Injection Yuelexin® Cefoxitin Sodium for Injection
Yuelexin® Cefoxitin Sodium for Injection Lilexin® Cefodizime Sodium for Injection
Lilexin® Cefodizime Sodium for Injection Ailexin® Cefpirome Sulfate for Injection
Ailexin® Cefpirome Sulfate for Injection